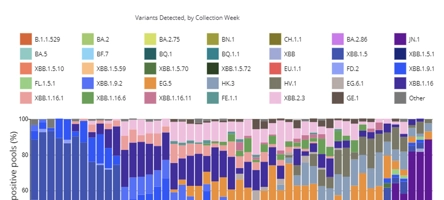
FAQ: Why Do I Keep Getting COVID?
The Latest

About the COVID-19 Real-Time Learning Network
The Real-Time Learning Network is a platform for sharing accurate, timely information about COVID-19. It has supported tens of thousands of clinicians who have engaged with the network since its creation in 2020. Our editorial team of infectious diseases and public health experts synthesizes clinical guidance, identifies emerging scientific consensus and areas of ongoing uncertainty, and tackles misconceptions and disinformation.