Infectious Diseases Society of America Guidelines on Infection Prevention for Healthcare Personnel Caring for Patients with Suspected or Known COVID-19
Published by IDSA on 4/27/2020. Last updated,
COVID-19 Guideline, Part 1: Treatment and Management
COVID-19 Guideline, Part 3: Molecular Testing
COVID-19 Guideline, Part 4: Serologic Testing
COVID-19 Guideline, Part 5: Antigen Testing
Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians
John B. Lynch*, Perica Davitkov**, Deverick J. Anderson, Adarsh Bhimraj, Vincent Chi-Chung Cheng, Judith Guzman-Cottrill, Jasmine Dhindsa, Abhijit Duggal, Mamta K. Jain, Grace M. Lee, Stephen Y. Liang, Allison McGeer, Jamie Varghese, Valéry Lavergne**, M. Hassan Murad**, Reem A. Mustafa**, Shahnaz Sultan**, Yngve Falck-Ytter**, Rebecca L. Morgan**
*Corresponding Author **Methodologist
November 4, 2021
Version 2.0.0 has been released and contains a new recommendation on the use of eye protection as well as revised recommendations and literature on the use of PPE. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
Update History
April 30, 2020
Language was added to clarify that an indirect study regarding aerosolized SARS-CoV-2 in negative pressure or airborne isolation rooms does not correlate with a human clinical setting (see last section of narrative summary).
Abstract
Background: Since its emergence in late 2019, SARS-CoV-2 continues to pose a risk to healthcare personnel (HCP) and patients in healthcare settings. Although all clinical interactions likely carry some risk of transmission, human actions like coughing and care activities like aerosol-generating procedures likely have a higher risk of transmission. The rapid emergence and global spread of SARS-CoV-2 continues to create significant challenges in healthcare facilities, particularly with shortages of personal protective equipment (PPE) used by HCP. Evidence-based recommendations for what PPE to use in conventional, contingency, and crisis standards of care continue to be needed. Where evidence is lacking, the development of specific research questions can help direct funders and investigators.
Objective: Develop evidence-based rapid guidelines intended to support HCP in their decisions about infection prevention when caring for patients with suspected or known COVID-19.
Methods: IDSA formed a multidisciplinary guideline panel including frontline clinicians, infectious disease specialists, experts in infection control, and guideline methodologists with representation from the disciplines of public health, medical microbiology, pediatrics, critical care medicine and gastroenterology. The process followed a rapid recommendation checklist. The panel prioritized questions and outcomes. Then a systematic review of the peer-reviewed and grey literature was conducted. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of evidence and make recommendations.
Results: The IDSA guideline panel agreed on eight recommendations, including two updated recommendations and one new recommendation added since the first version of the guideline. Narrative summaries of other interventions undergoing evaluations are also included.
Conclusions: Using a combination of direct and indirect evidence, the panel was able to provide recommendations for eight specific questions on the use of PPE for HCP providing care for patients with suspected or known COVID-19. Where evidence was lacking, attempts were made to provide potential avenues for investigation. There remain significant gaps in the understanding of the transmission dynamics of SARS-CoV-2 and PPE recommendations may need to be modified in response to new evidence. These recommendations should serve as a minimum for PPE use in healthcare facilities and do not preclude decisions based on local risk assessments or requirements of local health jurisdictions or other regulatory bodies.
Keywords: coronavirus, SARS-CoV-2, COVID, COVID-19, pneumonia, infection prevention
Executive Summary and Background
Executive Summary
Summarized below are the recommendations for infection prevention among healthcare personnel (HCP) caring for suspected or known patients with coronavirus disease 2019 (COVID-19). A detailed description of background, methods, evidence summary and rationale that support each recommendation, and research needs can be found online in the full text. In brief, per Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, recommendations are labeled as “strong” or “conditional”. The word “recommend” indicates strong recommendations, and “suggest” indicates conditional recommendations. In situations where the guideline panel judged there was insufficient evidence of benefit to support the use of specific personal protective equipment (PPE) with concerns for negatively impacting resources, the expert panel acknowledged the knowledge gap and made no recommendation, highlighting the need for more definitive evidence.
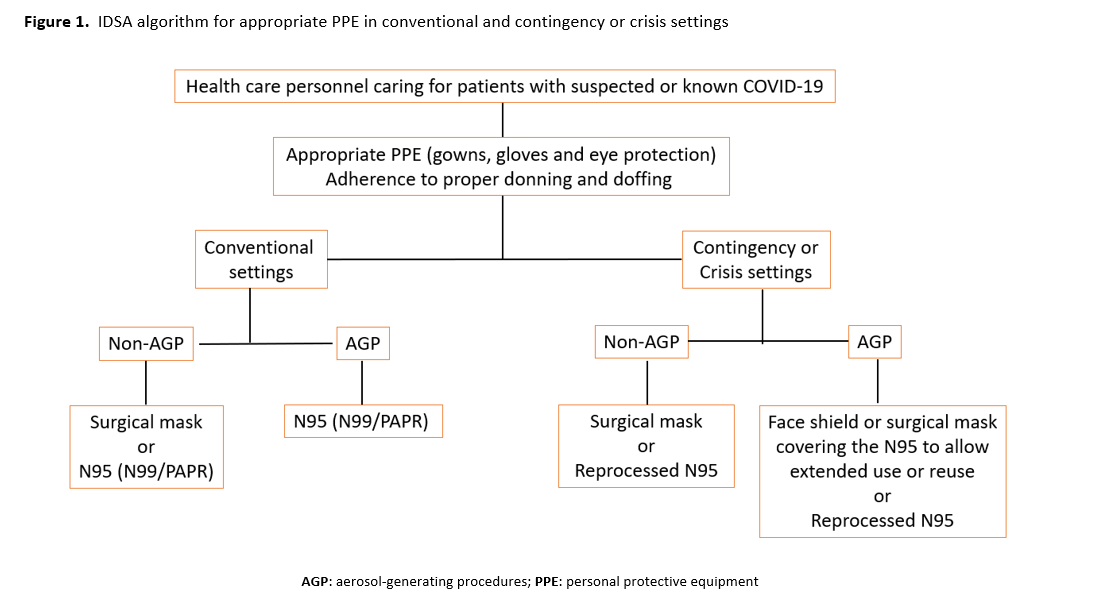
The IDSA guideline panel used the Crisis Standards of Care framework to develop its recommendations [1-3]. In the setting of a pandemic with documented shortages of PPE across various healthcare settings, the availability of supplies is an important driver of recommendations. Using the crisis capacity framework, separate recommendations were made for contingency or crisis capacity settings acknowledging the limited availability of PPE (Figure 1).
For all the recommendations below, the panel emphasizes the impact of conventional, contingency and crisis standards of care on how PPE is used. It also is critical to emphasize the importance of “appropriate PPE” for the care of patients with suspected or known COVID-19, including gowns and gloves, as well as adherence to standards for donning and doffing to minimize transmission. The panel recognizes the need to address the potential role of eye protection and masks as part of standard precautions, how to mitigate gown shortages (e.g., use of garbage bags as a safe alternative), and if there is a role for hair covers to prevent SARS-CoV-2 transmission. In addition, the behaviors, protocols, and environments associated with PPE use—particularly while PPE is being removed—cannot be separated from the technical qualities of the equipment. The panel hopes to address these questions in subsequent updates.
Recommendations
Routine Patient Care in CONVENTIONAL Settings
- Recommendation 1:The IDSA guideline panel recommends that healthcare personnel caring for patients with suspected or known COVID-19 use either a medical/surgical mask or N95 (or N99 or PAPR) respirator compared with no mask as part of appropriate PPE*. (Strong recommendation, moderate certainty of evidence)
- Recommendation 2: The IDSA guideline panel suggests that healthcare personnel caring for patients with suspected or known COVID-19 use eye protection compared with no eye protection as part of appropriate PPE*. (Conditional recommendation, very low certainty of evidence)
Routine Patient Care in CONVENTIONAL, CONTINGENCY, or CRISIS CAPACITY Settings
- Recommendation 3: The IDSA guideline panel makes no recommendation for the use of double gloves versus single gloves for healthcare PPE*. (Knowledge gap)
- Recommendation 4: The IDSA guideline panel makes no recommendation for the use of shoe covers versus no shoe covers for healthcare personnel caring for patients with suspected or known COVID-19 as part of appropriate PPE*. (Knowledge gap)
Aerosol-Generating Procedures in CONVENTIONAL Settings
- Recommendation 5: The IDSA guideline panel recommends that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients use an N95 (or N99 or PAPR) respirator instead of a medical/surgical mask, as part of appropriate PPE*. (Strong recommendation, very low certainty of evidence)
- Remark: Despite the very low quality and indirect evidence supporting this recommendation, the IDSA guideline panel placed a high value on avoiding serious harms to exposed healthcare personnel.
Aerosol-Generating Procedures in CONVENTIONAL, CONTINGENCY, or CRISIS CAPACITY Settings
- Recommendation 6: During contingency or crisis capacity settings (N95 respirator shortages), the IDSA guideline panel suggests that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients use a REPROCESSED N95 respirator for reuse instead of medical/surgical masks as part of appropriate PPE*. (Conditional recommendation, very low certainty evidence)
- Recommendation 7: During contingency or crisis settings (respirator shortages), the IDSA guideline panel recommends that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or medical/surgical mask as a cover for the N95 respirator to allow for EXTENDED use as part of appropriate PPE*. (Strong recommendation, very low certainty evidence).
- Remark: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or medical/surgical mask covering the respirator.
- Recommendation 8: During contingency or crisis settings (respirator shortages), the IDSA guideline panel suggests that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or medical/surgical mask as a cover for the N95 respirator to allow for REUSE as part of appropriate PPE*. (Conditional recommendation, very low certainty evidence).
- Remark: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or medical/surgical mask covering the respirator.
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Background
The first cases of coronavirus disease 2019 (COVID-19) were reported from Wuhan, China in early December 2019 [4], now known to be caused by a novel beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Within a span of months, COVID-19 became a pandemic due to its transmissibility, spreading across continents with the number of cases and deaths rising daily [5]. Although many infected individuals are asymptomatic or exhibit a mild illness, 20% have severe or critical illness requiring hospitalization and between 3% to 20% of hospitalized people die depending on severity of illness [6-8]. The mortality rate remains even higher for those patients requiring extracorporeal life support [4, 9-11]. Severity of illness is associated with systematic barriers to care (racial constructs and ethnicity), age, medical co-morbidities, and pregnancy [12, 13]. Transmission occurs from persons incubating the disease before the onset of symptoms, from persons with mild illness, and from persons with asymptomatic infection [14, 15]. Although viral shedding decreases soon after symptom onset, many patients shed non-infectious viral RNA for prolonged periods [16-18]. Throughout the course of infection, much remains unknown about the risk of transmission between patients and caregivers. The emergence of SARS-CoV-2 variants of concern, most notably the delta variant, and higher levels of transmission, create additional complexity as findings of prior studies may not be extrapolated to current or future conditions.
Accurately identifying the contribution to overall transmission of different modes of transmission of respiratory viruses has been a challenge. Over the last year, individuals and groups from different areas of science, most notably environmental aerosol researchers, have determined that the short-range continuum of small respiratory particles, ranging from aerosols to droplets, are likely the major contributors of transmission [19, 20]. These findings call into question the historical classification of precautions and associated PPE, most notably droplet (requiring medical/surgical masks) and airborne (requiring respirators) precautions. Globally, many—if not most—hospitals, clinics, and healthcare systems have been using droplet and contact precautions for patients with COVID not undergoing aerosol-generating procedures (AGPs) throughout the pandemic. The primary mechanism to reduce aerosol transmission is ventilation. Higher levels of air exchanges and filtration are commonly required in U.S. healthcare settings, especially hospitals [21]. Additionally, the decay in viral load following symptom onset likely means that by the time patients are hospitalized with moderate, severe, or critical illness, the risks of transmission are reduced [22, 23]. Studies that determine a biological basis of transmission risk within healthcare settings (as opposed to the current “AGP vs. non-AGP”) in the context of local transmission rates, ventilation, building design, screening and surveillance testing, patient-side source control, and likely other factors, continue to be needed to inform appropriate PPE recommendations. The role of PPE in prevention of transmission to patients also needs to be addressed.
Any guidance on PPE would be remiss not to mention the critical role of SARS-CoV-2 vaccination for HCP. The data supporting their efficacy, effectiveness, and safety are robust. Universal and required vaccination of HCP provides the most important level of protection, and when combined with appropriate PPE and other interventions, creates the safest possible environment for HCP providing care for patients with COVID-19.
Given the rapid global spread of SARS-CoV-2 and the difficulties faced by overburdened frontline HCP and policymakers trying to remain up to date on emerging literature, IDSA has recognized the necessity of developing a rapid guideline for infection prevention in healthcare settings. The guideline panel used a methodologically rigorous process for evaluating the best available evidence and providing treatment recommendations. A limited number of specific questions were chosen for review based on recommendations from the panel members, all of whom are currently working directly with patients with COVID-19 and/or on policies and protocols for the healthcare response.
This guideline on infection prevention complements IDSA’s four other guidelines on COVID-19 addressing treatment and management, molecular diagnostic testing, serologic testing, and antigen testing. These guidelines will be regularly updated with questions added as substantive literature becomes available and will be made accessible on an easy to navigate web and device interface: http://www.idsociety.org/covid19guidelines/ip.
These recommendations are intended to inform patients, clinicians, and other HCP by providing the latest available evidence.
Aerosol-generating procedures
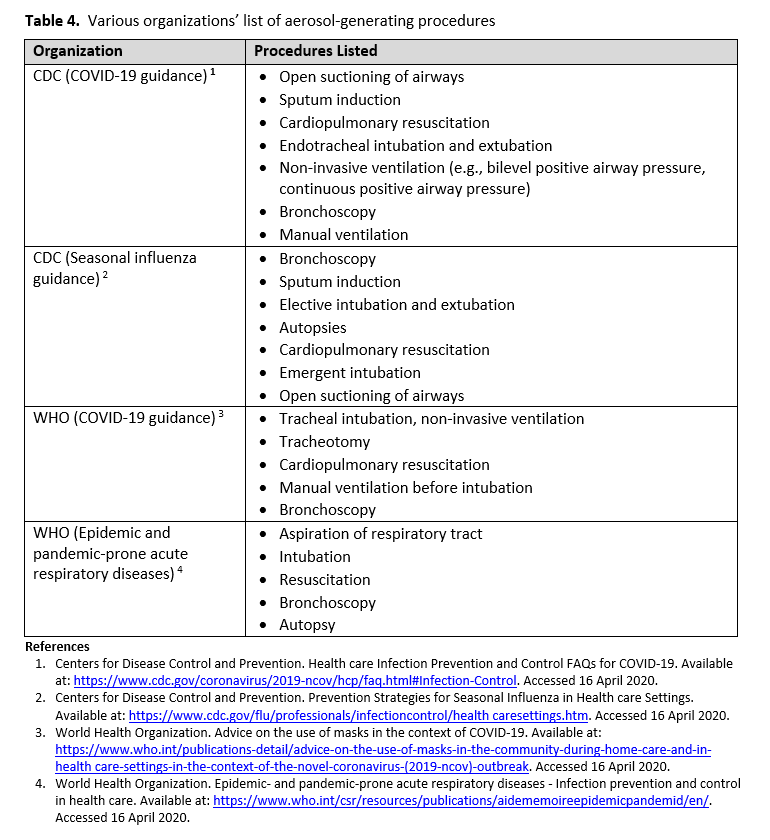
Person-to-person transmission of SARS-CoV-2 virus occurs primarily via exposure to an infectious person’s respiratory droplets. Respiratory protection (i.e., surgical mask) prevents droplets from contacting the mucous membranes of a person’s nose and mouth. Some medical procedures are more likely to generate higher concentrations of infectious small particle (<0.5 μm) respiratory aerosols. These procedures, referred to as AGPs, could potentially increase HCP exposure risk to SARS-CoV-2 (see Table 4 for the lists of AGPs from various organizations). Thus, a higher level of respiratory protection may be needed to protect HCP from inhaling smaller aerosolized particles. N95 and higher-level respirators, such as disposable FFRs, PAPRs, and elastomeric respirators provide additional protection due to their filtering capabilities. As with droplet transmission, eye protection in the form of goggles or face shield, is required.
Methods
This guideline was developed using the GRADE approach for evidence assessment. In addition, given the need for an urgent response to a major public health crisis, the methodological approach was modified according to the Guideline International Network/McMaster checklist for the development of rapid recommendations [24].
Panel composition
The initial guideline panel assembled in March 2020 was composed of eleven members including frontline clinicians, infectious disease specialists, experts in infection prevention and guideline methodologists. The panel also included experts in preventive care, public health, medical microbiology, pediatrics, critical care medicine and gastroenterology. Organizational representatives were included on the panel from the Society for Healthcare Epidemiology of America (SHEA) and the Pediatric Infectious Diseases Society (PIDS). The Evidence Foundation provided technical support and guideline methodologists for the development of this guideline.
Disclosure and management of potential conflicts of interest
The conflict of interest (COI) review group included two representatives from IDSA who were responsible for reviewing, evaluating, and approving all disclosures. All members of the expert panel complied with the COI process for reviewing and managing conflicts of interest, which required disclosure of any financial, intellectual, or other interest that might be construed as constituting an actual, potential, or apparent conflict, regardless of relevancy to the guideline topic. The assessment of disclosed relationships for possible COI was based on the relative weight of the financial relationship (i.e., monetary amount) and the relevance of the relationship (i.e., the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The COI review group ensured that the majority of the panel and chair were without potential relevant (related to the topic) conflicts. The chair and all members of the technical team were determined to be unconflicted.
Question generation
Clinical questions were developed into a PICO format (Population, Intervention, Comparison, Outcomes) [25] prior to the first panel meeting. For these guidelines to be implementable in various healthcare environments, two types of clinical settings were defined a priori to account for the availability of PPE: 1) conventional settings (i.e., no restriction on PPE availability), 2) contingency or crisis capacity settings (i.e., limited availability of PPE). Panel members focused on the protective effect of PPE on HCP such as the prevention of healthcare-associated transmission of viral respiratory infections (RVI) (either laboratory-confirmed infection or inferred by clinical compatible syndrome), and adverse events leading to discontinuation of PPE.
This guideline iteration addressed the following PICO questions:
- Should HCP caring for persons with suspected or known COVID-19 use either a medical/surgical mask or respirator—rather than no mask—as part of appropriate PPE*?
- During a contingency or crisis capacity setting (N95 respirator shortages), should HCP involved with AGPs on suspected or known COVID-19 patients use a reprocessed N95 respirator for reuse rather than a medical/surgical mask, as part of appropriate PPE*?
- Should HCP caring for persons with suspected or known COVID-19 use eye protection rather than no eye protection, as part of appropriate PPE*?
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Search strategy
With the help of an information specialist, OVID Medline, EMBASE, and Cochrane Library were searched to identify all relevant English studies from inception to March 8, 2021 related to COVID-19 (Supplementary Table s1). In certain circumstances, searches were also conducted to identify relevant literature using Google Scholar and the World Health Organization (WHO) and U.S. Centers for Disease Control and Prevention (CDC) websites. Horizon scans were performed routinely during the evidence assessment and recommendation process to locate additional grey literature and manuscript pre-prints. Reference lists and literature suggested by panelists were reviewed for inclusion. The reference lists of relevant articles were scanned for additional studies.
When applicable, existing systematic reviews were used to inform the recommendations. Laboratory experiments were also reviewed to provide further indirect evidence of mechanistic explanations when appropriate.
Screening and study selection
Two reviewers independently screened titles and abstracts as well as eligible full-text studies. When acceptable systematic reviews were identified (i.e., recent, well-done systematic reviews of randomized controlled trials [RCTs] or comparative non-randomized studies), no additional RCTs or comparative non-randomized studies were sought. When acceptable RCTs of effectiveness were found, no additional non-randomized studies or non-comparative evidence (i.e., single-arm case series) were sought. Evidence from experimental or laboratory studies were included as sources of indirect evidence and public health websites, such as the CDC and WHO, were also reviewed for additional literature.
Data collection and analysis
Pairs of reviewers extracted relevant information into a standardized data extraction form. Existing systematic reviews also were reviewed for methodologic rigor [26]. When appropriate, specific subgroup analyses were conducted using Review Manager [27].
Risk of bias and certainty of evidence
Reviewers assessed risk of bias with the Cochrane Risk of Bias Tool for RCTs and the Risk of Bias Instrument for Non-randomized Studies – of Interventions (ROBINS-I) [26, 28].
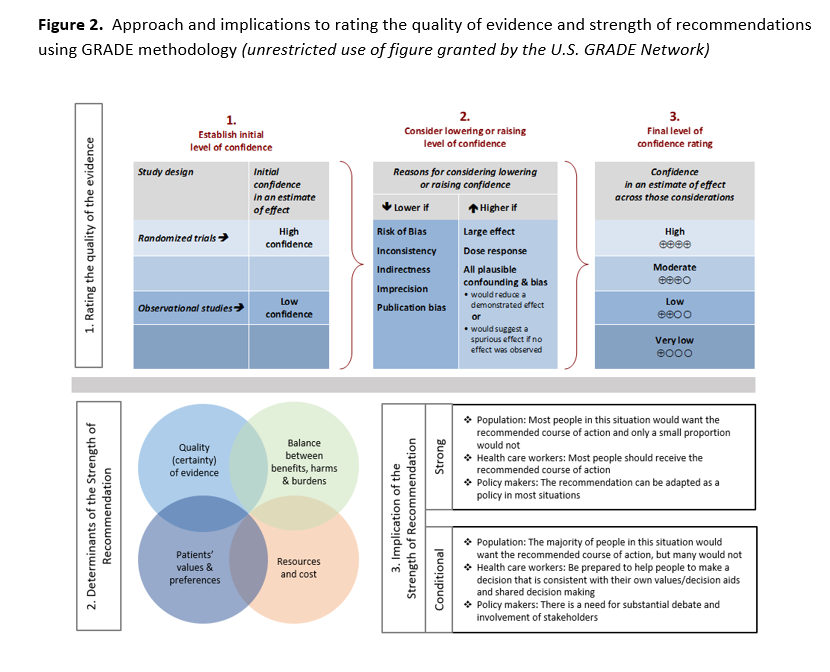
The certainty of evidence was assessed using the GRADE approach [29]. Within GRADE, the body of evidence across each outcome is assessed for domains that may reduce or increase one’s certainty in the evidence (Figure 2). Evidence from RCTs start as high-certainty evidence and observational studies start out as low-certainty of evidence. Factors that may reduce one’s certainty include risk of bias (study limitations), inconsistency (unexplained heterogeneity across study findings), indirectness (applicability or generalizability to the research question), imprecision (the confidence in the estimate of an effect to support a particular decision), or publication bias (selective publication of studies). One’s certainty in the evidence may be strengthened if the following considerations are present: large or very large magnitude of effect, evidence of a dose-response gradient, or opposing residual confounding. The certainty of evidence is categorized into four levels ranging from very low to high. For each recommendation, an overall judgment of certainty of evidence is made based on critical outcomes.
Evidence profiles were used to display the summary estimates as well as the judgments about the overall certainty of the body of evidence for each clinical question across outcomes. GRADE evidence profiles and summary of findings tables were developed in GRADEpro Guideline Development Tool [30].
Evidence to recommendations
The panel considered core elements of the GRADE evidence in the decision process, including certainty of evidence, balance between desirable and undesirable effects, assumption on values and preferences, and resource implications. Additional domains were acknowledged where applicable (equity, acceptability, and feasibility). The panel deliberated over the impact of resource limitations on the feasibility of and ability to implement these recommendations. Therefore, the panel’s recommendations addressed both “conventional” settings where there is no restriction on PPE availability and “contingency or crisis capacity” settings in which PPE availability is limited.
For all recommendations, the expert panelists reached consensus. Voting rules were agreed on prior to the panel meetings for situations when consensus could not be reached. As per GRADE methodology, recommendations are labeled as “strong” or “weak/conditional”. The words “we recommend” indicate strong recommendations and “we suggest” indicate conditional recommendations. Figure 2 provides the suggested interpretation of strong and weak/conditional recommendations for patients, clinicians, and healthcare policymakers. In some situations where the evidence was judged insufficient to provide a clear direction “for” or “against" a particular management strategy, the panel decided to make a “no recommendation”.
According to the GRADE approach, strong recommendations in the setting of lower-quality evidence were only assigned when the panelists believed they conformed to one or several paradigmatic conditions. As per GRADE guidance [31] on discordant recommendations, there are five paradigmatic situations that can be conceptualized as ones in which there are clear benefits in the setting of a life-threatening situation, clear catastrophic harms, or equivalence between two interventions with clear harms for one of the alternatives.
Although there is ongoing need for research on virtually all of the topics considered in this guideline, “Research Needs” were noted for recommendations in which the need was believed by the panelists to be particularly relevant.
Review process
This guideline underwent a rapid review for approval by IDSA Board of Directors Executive Committee external to the guideline development panel. The IDSA Board of Directors Executive Committee reviewed and approved the guideline prior to dissemination. The guideline was reviewed and endorsed by SHEA and PIDS.
Updating process
Regular, frequent screening of the literature will take place to determine the need for revisions based on the likelihood that any new data will have an impact on the recommendations. When necessary, the entire expert panel is reconvened to discuss potential changes.
Definitions and Search Results
Definitions
Medical/surgical masks: Masks with or without plastic shields are used as a physical barrier to protect the user from hazards, such larger respiratory droplets. Medical masks also protect other people against infection from the person wearing the medical mask. Such masks trap large particles of body fluids that may contain bacteria or viruses expelled by the wearer [32]. Surgical masks and medical masks are used interchangeably in this document as the fluid impermeable rating (associated with surgical masks specifically) was not relevant.
Respirator: Devices used to protect healthcare personnel from airborne particles that can lead to infection. This includes N95 filtering facepiece respirators (FFRs) and higher-level “mask-like” respirators (e.g., N99, N100), powered air purifying respirators (PAPRs), controlled air purifying respirators, and elastomeric respirators.
Donning and doffing procedures: The practice of putting on and removing PPE. Donning refers to putting on PPE, while doffing means removing it.
Crisis standards of care [1]:
- Conventional capacity: Usual supplies available and used
- Contingency capacity: Conservation, adaptation and substitution of supplies with occasional reuse of select supplies
- Crisis capacity: Critical supplies lacking
PPE extended use: The use of PPE for greater than a single patient encounter and without removing the PPE, with or without the use of additional devices (e.g., a face shield over a surgical mask). Recommended for use only in contingency or crisis capacities [3].
PPE reuse: The use of PPE that is doffed after each patient encounter and re-donned after a period of time and/or a processing step. Recommended for use only in contingency or crisis capacities [3].
Search results
Mask use: Systematic review and horizon scan of the literature identified 2,789 references of which nine informed the evidence base of the recommendation on eye protection (Supplementary Figure s1).
Mask reuse: Systematic review and horizon scan of the literature identified 487 references of which 19 informed the updated evidence base of the recommendation on mask reuse (Supplementary Figure s2).
Eye protection: Systematic review and horizon scan of the literature identified 1,116 references of which five informed the evidence base of the recommendation on eye protection (Supplementary Figure s3).
For all recommendations below, the panel emphasizes the importance of “appropriate PPE” (which includes gowns, gloves, and eye protection) and adherence to standards for donning and doffing to minimize transmission.
Recommendations 1 and 2: Routine patient care in CONVENTIONAL settings
Medical/surgical mask or N95 respirator
Recommendation 1: The IDSA guideline panel recommends that healthcare personnel caring for patients with suspected or known COVID-19 use either a medical/surgical mask or N95 (or N99 or PAPR) respirator compared with no mask as part of appropriate PPE*. (Strong recommendation, moderate certainty of evidence)
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
No direct comparative data from RCTs was identified. However, our search identified direct evidence from observational comparative cohort and case-control studies. We excluded non-comparative studies, case reports or summary of series case reports with which we were unable to identify a denominator. We also excluded studies in which authors were unable to ascertain a reliable COVID-19 diagnosis or studies that were done in community settings.
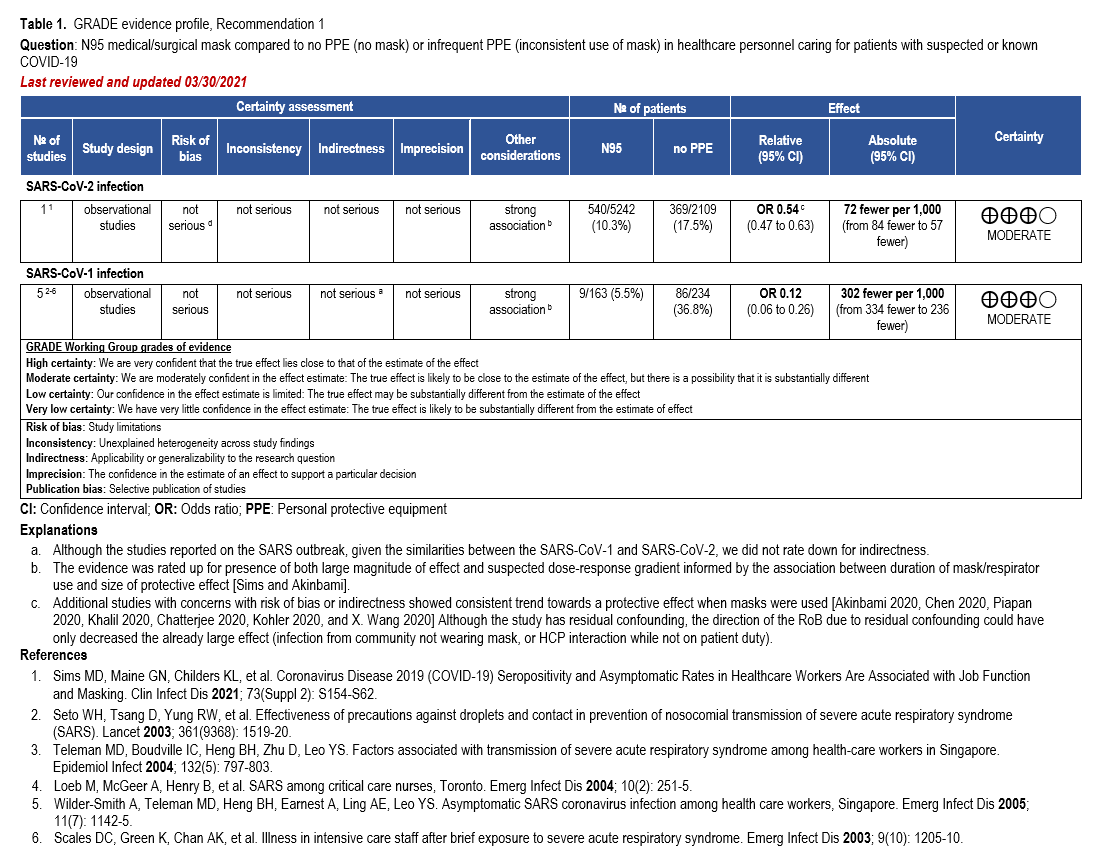
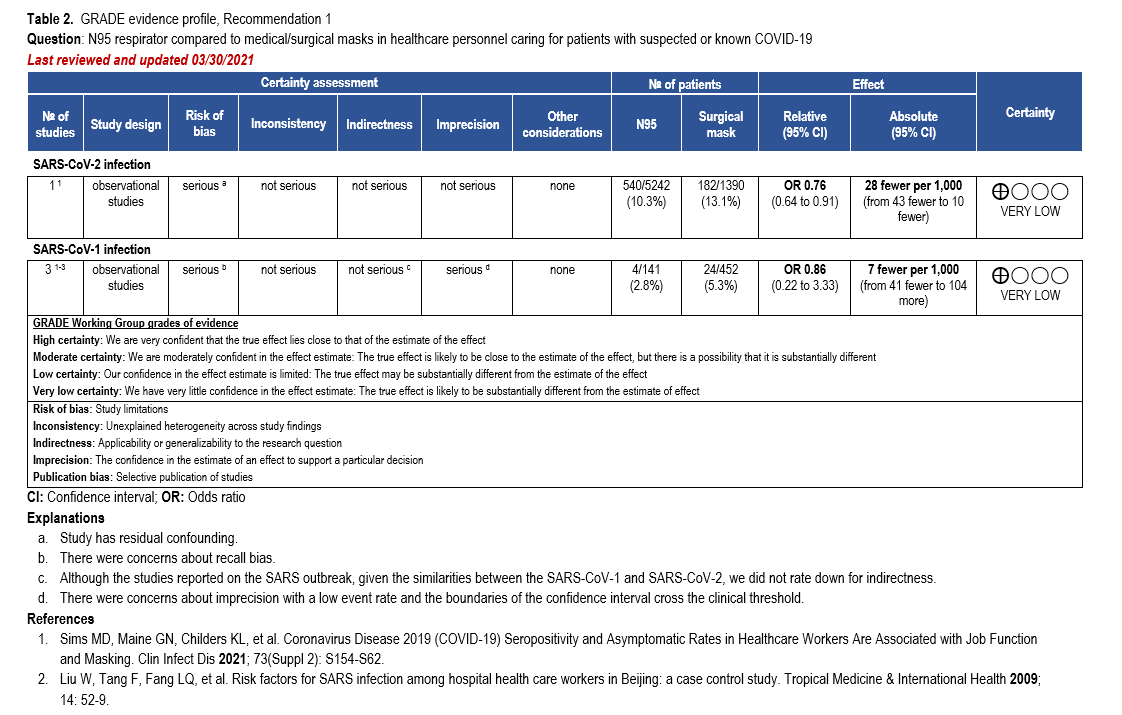
The body of evidence was too heterogeneous to pool quantitatively in a meta-analysis due to differences with risk of bias, study setting, type of mask assessed, and ascertainment of the exposure and outcomes. Thus, we decided to present the best available evidence coming from a single study [33] in an evidence profile along with the previous analysis from SARS-CoV-1. This was a large cross-sectional, comparative study of 20,614 HCP (e.g., frontline workers such as nurses, respiratory therapists, and physicians who had direct contact with patients with COVID-19, as well as support staff) in which a questionnaire was used to identify HCP behavior including PPE use, and a SARS-CoV-2 IgG assay was performed to confirm COVID-19 serostatus. Healthcare personnel wore surgical masks or N95/PAPR. Of those wearing any type of mask, 10.9% (721/6624) were seropositive, compared to 17.5% (369/2109) of those not wearing any type of mask (odds ratio [OR]: 0.54; 95% CI: 0.47 to 0.63). Furthermore, of those wearing N95/PAPR, 10.3% (540/5242) were seropositive compared with 13.1% (182/1390) of those wearing surgical masks (OR 0.76; 95% CI: 0.64 to 0.91) (Tables 1-2).
All other identified studies demonstrated a consistent trend toward a protective effect when mask or N95 respirator was used. Similarly, a comparative cross-sectional study used a web survey to analyze samples from 16,397 adults working in first response, hospital, or public safety settings [34]. Consistent N95 respirator wearing was associated with decrease infection rates when compared to sometimes wearing an N95 respirator (adjusted OR [aOR]: 0.83; 95% CI: 0.72, 0.95). Results were same for use of surgical facemasks ‘all the time’ compared to ‘sometimes’ (aOR: 0.86; 95% CI: 0.75, 0.98). The authors of this study adjusted their analysis for sex, age group, race, location, PPE, housing, AGPs, exposure to person with COVID-19 (co-worker, household, patient), occupation, and workplace (emergency department, intensive care unit [ICU], fire service, police). Four additional smaller cross-sectional studies were identified. One study surveyed 105 HCP who were exposed to patients with COVID-19 [35]. There were 18 seropositive close contacts, of which 10 wore disposable non-surgical face masks. There were also 87 seronegative close contacts, of which 68 wore disposable non-surgical face masks (aOR: 0.13; 95% CI: 0.02, 0.97). The second study interviewed 903 HCP who reported contact with patients with COVID-19 [36]. One-hundred fifteen HCP tested positive for COVID-19, of whom 50 (44%) used surgical masks. A total of 788 HCP tested negative for COVID-19; however, data was only available for 144 HCP, of whom 91 (63%) wore a surgical mask. In the third study, out of 138 HCP tested, seven were positive for COVID-19. Of those, 15% wore an N95 mask for every patient visit compared to 29% wearing N95 from the 131 HCP testing negative for COVID-19 [37]. In the fourth study of 3,064 emergency department providers, 164/3064 (5.1%) tested positive for SARS-CoV-2. Of this population, 73/944 (7.3%) were from “high level of protection hospitals” (i.e., hospitals where everybody wore FFP2 masks or equivalent) and 91/2099 (4.0%) from “other hospitals” (i.e., hospitals where some providers did not wear FFP2 masks or equivalent). However, the adjusted analysis for a hospital testing policy reported no statistical difference in infection rates between the hospitals [38].
Our research also identified two case-control studies reporting on interview schedules and questionnaires of HCP. In Khalil 2020 [39], 98 physicians testing positive for COVID-19 were matched with 92 physicians identified as negative for COVID-19 (i.e., physicians with no symptoms or who tested negative and had known or unknown interactions with COVID-19 positive patients). Of the HCP who tested positive, 95.5% (85/89) wore unspecified N95 masks. Of the HCP who tested negative, 96.7% (89/92) wore unspecified N95 masks. The second case-control study looked at outcomes of unspecified mask use compared with non-mask use [40]. Symptomatic HCP who tested positive on PCR were considered cases, while symptomatic HCP who tested negative were deemed controls. In the control group, 92.76% (346/373) reported wearing masks; in the case group, only 82% (310/378) reported wearing masks.
Lastly, three retrospective cohort studies were identified. In Kohler 2020 [41], 1,012 hospital workers who had antibody (Ab) testing were followed. Of those, three of 10 (30%) who tested positive wore a mask, while 562 of 1,002 (71.1%) people who tested negative wore a mask (OR 0.17; 95% CI: 0.04, 0.68). In X. Wang 2020 [42], researchers looked at a total of 493 medical staff. Two-hundred seventy-eight staff members were in the departments of Respiratory Medicine, ICU, and Infectious Diseases and wore N95s, while 213 staff members in Hepatobiliary Pancreatic Surgery, Trauma, Microsurgery, and Urology did not wear masks at all. Of those reporting wearing N95s, 0/278 (0%) staff members became infected with COVID-19 compared with 10/213 (4.7%) staff who did not wear masks. The third study used a pre-post design with the introduction of a hygiene bundle that included a mandatory surgical mask. A total of 1,061 patients were evaluated before intervention, while 5,045 patients used the hygiene bundle. Twenty-seven patients (13.7%) in the standard protocol were confirmed with SARS-CoV-2 while 86 patients (10.3%) in the hygiene bundle were confirmed with SARS-CoV-2 (OR 0.70: 95% CI 0.44, 1.11) [43].
Other considerations
Evidence to support the use of N95 respirators or surgical masks (as compared with no masks or infrequent mask use) is derived from observational studies. The best evidence come from a single comparative cohort study that was rated up for presence of both large magnitude of effect and suspected dose-response gradient informed by the association between duration of mask/respirator use and size of protective effect. Although the study had some concerns with residual confounding, the direction of the risk of bias due to residual confounding could have only decreased the already large effect (e.g., infection from community not wearing mask, or HCP interaction while not on patient duty) (Table 1). Thus, the overall certainty of evidence was moderate. Additional studies with concerns with risk of bias or indirectness showed consistent trend towards a protective effect when masks were used.
Conclusions and research needs for this recommendation
The guideline panel recommends that HCP caring for confirmed or suspected COVID-19 patients use a medical mask or an N95. Comparative data for use of N95 versus medical mask was limited. Additional well-designed RCTs or prospective cohort studies with appropriate active comparison groups are needed to address the potential superiority of N95 or higher-grade respirators compared with surgical masks in HCP taking care of patients with COVID-19.
Current CDC and U.S Occupational Safety and Health Administration (OSHA) guidelines and regulations indicate that HCP use respirators for the care of patients with known or suspected COVID-19. This guidance supersedes the evidence-based recommendation in this document for healthcare facilities in the United States. The approach used and recommendations made by the IDSA guideline panel highlights the need for additional research to address this important gap. Like other workplace hazards, PPE does not function independently of other risk reduction interventions, including ventilation and filtration, testing, and vaccination. Unlike other workplace hazards, epidemic infectious diseases also put HCP at risk when away from patients (e.g., break rooms) and away from the workplace, which creates challenges when evaluating the impact of an intervention like PPE. The biology of COVID-19, especially viral loads in the airway of affected individuals, creates additional confounders for evaluation.
Eye protection
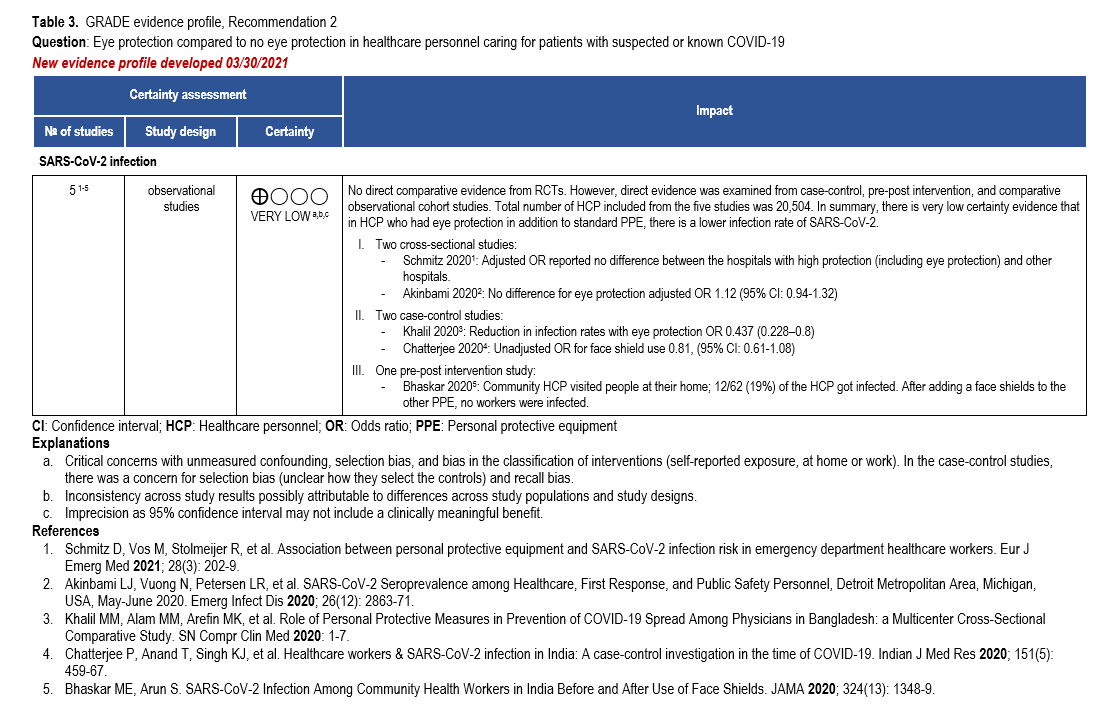
Recommendation 2: The IDSA guideline panel suggests that healthcare personnel caring for patients with suspected or known COVID-19 use eye protection compared with no eye protection as part of appropriate PPE*. (Conditional recommendation, very low certainty of evidence)
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
Our search identified direct comparative evidence reporting on eye protection from case-control, pre-post intervention, cross-sectional and cohort studies. We did not identify any comparative evidence reporting on COVID-19 infection rates with or without eye protection from RCTs. Lab experiments and single arm cohort studies without a comparison arm were excluded.
Two cross-sectional studies used standardized online questionnaires to measure eye protection use and SARS-CoV-2 infection rates in HCP [34, 38]. One observational study from the Netherlands [38], included 3,064 emergency department providers, of whom 164/3064 (5.1%) tested positive for SARS-CoV-2. Of this population, 73/944 (7.3%) were from “High level of protection hospitals” (i.e., hospitals where everybody wore eye protection) and 91/2099 (4.0%) from the “Other hospitals” (i.e., hospitals where some providers did not wear eye protection). The adjusted analysis reported no statistical difference in infection rates between the hospitals. The second cross-sectional study from Detroit, MI, reported 1131/16,397 (6.9%) HCP positive for SARS-CoV-2. Similarly, there was no difference for eye protection within the adjusted analysis (OR: 1.12; 95% CI: 0.94, 1.32) [34].
In addition, two case-control studies [39, 40] surveyed or interviewed HCP with confirmed SARS-CoV-2 (cases) and matched them with SARS-CoV-2-negative HCP from the same healthcare system (controls) during usual patient care. In one study, 55/92 (59.8 %) of the COVID-19 positive physicians wore face-shield/goggles compared to 68/88 (77.3%) of the COVID-19 negative physicians (OR 0.44; 95% CI: 0.228–0.8). During AGPs, 39/55 (70.9%) of the COVID-19 positive physicians reported wearing eye protection compared to 52/67 (77.6%) of the COVID-19 negative physicians (OR 0.702; 95% CI: 0.310–1.593) [39]. The second study of 378 cases and 373 controls, reported a decrease in infection among persons wearing face shields (OR: 0.81; 95% CI: 0.61-1.08) [40].
Lastly, in a pre-post intervention study [44], 112 community HCP were assigned to counsel asymptomatic family contacts of patients who had tested positive for SARS-CoV-2 at their residence. After visits to 31,164 patients with COVID-19, 12/62 (19%) of the HCP reported positive for SARS-CoV-2. After adding face shields to standard PPE, 0/50 HCP reported positive for SARS-CoV-2 following visits to 118,428 patients (2682 of whom were positive for SARS-CoV-2).
Other considerations
Evidence to support the use of eye protection (as compared with no eye protection) is derived from observational studies. Furthermore, those studies had critical concerns for risk of bias such as selection bias, bias in the classification of the intervention, and recall bias. Additionally, the body of evidence that informed the recommendation was too heterogenous to pool quantitatively in a meta-analysis; therefore, a narrative synthesis of the data was performed (Table 3). The overall quality of evidence was very low due to concerns with risk of bias, inconsistency, and imprecision. While the certainty of the evidence was determined to be very low, the panel recognized that even with a putative benefit, the addition of eye protection to PPE does not represent a substantial use of resources; however, the panel noted a potential undesirable consequence might be an increase in hand-to-face contact.
Conclusions and research needs for this recommendation
In summary, there is very low certainty evidence of a reduction in SARS-CoV-2 infection rates among HCP who wear eye protection, in addition to standard PPE. This recommendation was informed by observational studies that either demonstrated a modest reduction or no meaningful reduction in the risk of SARS-CoV-2 infection. However, in addition to this modest reduction in infection, the guideline panel determined that there is unlikely to be harm in the addition of eye protection to standard PPE.
The guideline panel recommends that HCP caring for confirmed or suspected COVID-19 patients use eye protection in conjunction with other recommended PPE and appropriate hand hygiene. The guideline panel also recognizes that additional well-designed studies are needed. Ideally, the evidence should come from well-designed pragmatic cluster RCTs that, for example, compare consistent eye protection in a controlled environment to current eye protection practices. Similarly, large prospective cohort studies with appropriate comparison groups and integration with prospective outcome registries are needed to address the potential superiority of eye protection compared to no eye protection in HCP caring for suspected or confirmed COVID-19 patients. Lastly, infectivity from community settings should be considered and adjusted for when designing studies addressing eye protection.
Recommendations 3 and 4: Routine patient care in CONVENTIONAL, CONTINGENCY, or CRISIS CAPACITY settings
Gloves
Recommendation 3: The IDSA guideline panel makes no recommendation for the use of double gloves versus single gloves for healthcare PPE*. (Knowledge gap)
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
There were no comparative studies that compared double versus single gloves to decrease infection rates. However, there is a theoretical risk of organism transfer from contaminated PPE to hands after removal of the contaminated gloves or clothing that may contribute to infection. In one study, swabs from 30 HCP PPE were collected after they exited COVID-19 patient rooms. There were no positive samples out of 90 collected swabs [45]. Furthermore, in a laboratory experiment simulating droplet contamination [46] two groups of participants were contaminated with bacteriophage MS2 after both groups donned a full set of PPE as per CDC guidance [47]one pair of gloves over the gown sleeve. The second group donned two pairs of latex gloves. The first (inner) pair of gloves was applied under the gown sleeve and the second (outer) pair was placed over the first pair positioned over the gown sleeve. During the doffing phase, the inner pair of gloves was removed last. The double-glove strategy was associated with less contamination than the single-glove strategy [46]. However, there was no report of hand hygiene or use of hand sanitizer between doffing sequences in the one glove group as per CDC recommendations which may have decreased the contamination in the single glove group [47]. A Cochrane systematic review found that contamination of body parts with MS2 virus was less with the use of double gloves than with single gloves, but the contamination of body parts as detected with fluorescent marker was not different [48].
Other considerations
The panel determined that there was insufficient evidence to make a recommendation on the use of double gloves.
Conclusions and research needs for this recommendation
There is conflicting indirect and experimental evidence on the importance of double gloving as a component of appropriate PPE when caring for a patient with suspected or known COVID-19. Further studies are needed to compare different glove doffing strategies to prevent infection in HCP performing usual care on COVID-19 patients.
Shoe covers
Recommendation 4: The IDSA guideline panel makes no recommendation for the use of shoe covers versus no shoe covers for healthcare personnel caring for patients with suspected or known COVID-19 as part of appropriate PPE*. (Knowledge gap)
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
There were no studies that evaluated shoe covers as part of routine PPE and COVID-19 transmission. In the study by Ong et al, HCP PPE was swabbed (~90 swabs obtained) after they exited COVID-19 patient rooms. Only one PPE swab obtained from the surface of a shoe front was found positive [45].
Other considerations
There were no studies that evaluated shoe covers as part of routine PPE and COVID-19 transmission. In the study by Ong et al, HCP PPE was swabbed (~90 swabs obtained) after they exited COVID-19 patient rooms. Only one PPE swab obtained from the surface of a shoe front was found positive [45].
Conclusions and research needs for this recommendation
Current guidance on PPE endorses the use of shoe covers when there is concern for splash risk from fluids that may contain pathogens. Further studies are needed to determine if shoe covers have any role in protecting HCP from infection in the context of COVID-19.
Recommendation 5: Aerosol-generating procedures in CONVENTIONAL settings
N95 respirator
Recommendation 5: The IDSA guideline panel recommends that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients use an N95 (or N99 or PAPR) respirator instead of a medical/surgical mask, as part of appropriate PPE*. (Strong recommendation, very low certainty of evidence)
- Remark: Despite the very low quality and indirect evidence supporting this recommendation, the IDSA guideline panel placed a high value on avoiding serious harms to exposed healthcare personnel.
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
There was no direct evidence on AGPs and rates of COVID-19 infection among HCP. Indirect evidence from the SARS epidemic was used to inform this recommendation. Based on observational data, among infected HCP with SARS, exposure to an AGP such as tracheal intubation was associated with a higher risk of infection (see Table 5) [49]. Evidence from laboratory simulation data also provided indirect evidence on the viability of aerosolized SARS-CoV-2 [50]. Additionally, data on environmental contamination was obtained by sampling various surfaces and air samples from confirmed COVID-19 patient rooms: 87% (13/15) room sites (including air exhaust outlet fans) returned positive SARS-CoV-2 on RT-PCR results and 60% (3/5) toilet sites (including toilet bowl, sink, and door handle) returned positive SARS-CoV-2 on RT-PCR results. Air samples were negative despite the extent of environmental contamination [51].
Other considerations
Evidence to support the use of N95 or higher-level respirators instead of surgical masks for HCP involved in AGPs was based on observational studies and experimental laboratory data. The overall certainty of evidence was very low due to limitations in the retrospective observational data and recall bias. However, the IDSA guideline panel made a strong recommendation for N95 or higher-level respirators, placing a high value on preventing infection among HCP.
Conclusions and research needs for this recommendation
The guideline panel recommends that when an AGP is being performed on a patient with suspected or known COVID-19, all involved HCP should wear an N95 or higher-level respirator, in addition to a gown, gloves and eye protection. Additional clinical studies are needed to inform our understanding of SARS-CoV-2 respiratory transmission in the healthcare setting. Studies are especially needed to clarify which medical procedures require a higher level of respiratory protection.
Recommendations 6-8: Aerosol-generating procedures in CONVENTIONAL, CONTINGENCY, or CRISIS CAPACITY settings
Reprocessed N95 respirator
Recommendation 6: During contingency or crisis capacity settings (N95 respirator shortages), the IDSA guideline panel suggests that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients use a REPROCESSED N95 respirator for reuse instead of medical/surgical masks as part of appropriate PPE*. (Conditional recommendation, very low certainty evidence)
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
We did not identify any comparative evidence from RCTs or cohort studies reporting on the infection rates of HCP using reprocessed N95 respirators rather than new or unprocessed N95 respirators, nor did we identify any direct evidence on infection rates using reprocessed masks. However, one cohort study reported an increased risk of positive COVID-19 tests among HCP who reported reuse of PPE compared to adequate PPE use (adjusted hazard ratio: 1.46; 95% CI: 1.21-1.76) [52]. This cohort study of the general community, including 99,795 frontline HCP, collected self-reported information through the COVID Symptom Study Smartphone application.
Furthermore, indirect evidence is available on re-processing of N95s utilizing ultraviolet (UV) radiation, heat, 70% ethanol and vaporized hydrogen peroxide (VHP) and reuse of N95s. Studies measured fitting factors by using PortaCount equipment for fit testing, which assessed the number of cycles that the N95 can be reused after re-processing without failing the fit test (Supplementary Table s6).
Six studies using hydrogen peroxide (HP) were identified, of which four used VHP [53-56], one used plasma HP [57], and one used ionized HP [58]. Studies measured filtration efficiency by assessing for ability to filter particles down to 0.3 μm using aerosolized sodium chloride challenge by National Institute for Occupational Safety and Health (NIOSH) precertification, direct measurement, or detection of the SARS-CoV-2. They also measured fit factor using quantitative fit testing with PortaCount equipment after respirator use and HP decontamination. Most of the studies conclude that all N95 respirator models can be decontaminated and reused up to three cycles. Similar conclusions supporting effective filtration and adequate fit factor with reuse of respirators after three cycles came from two studies on UV electromagnetic radiation decontamination [53, 59].
Similarly, heat with moisture generated by steam sterilization in three studies [60-62], by 50% relative humidity in one study [63], and by moisture alone (85 ˚C, 60-85% humidity) in one study [64] supported safe reuse after decontamination in up to three cycles.
Decontamination using dry heat administered at temperatures of 70-121 °C used in four studies [53, 63, 65, 66] and 70% ethanol used in one study [53] demonstrated acceptable filtration efficacy; however, respirator fit was impaired after one to two reuse cycles. In addition, those studies did not use decontamination methods that were NIOSH compliant.
Other considerations
No studies directly compared reprocessed N95 respirators with new or unprocessed N95 respirators, nor did any report on infection rates using reprocessed masks. Evidence informing this recommendation is from one cohort study that relied on self-report of PPE. Concerns with the certainty of the evidence included risk of bias and indirectness. Concerns with risk of bias included lack of adjustment for HCP behavior outside of the healthcare setting, as well as recall bias from self-reported data collection. The comparison of PPE reuse to adequate PPE use was indirect to the research question. In addition, the study did not report on the length of respirator use or number of cycles of respirator use. Additional indirect evidence came from decontamination methods studies that assessed mask integrity, filtration efficiency, and fit performance; however, none of these studies provided comparisons of reprocessed N95 respirators with new or unprocessed N95 respirators, and no direct evidence on infection rates using reprocessed masks was identified. Thus, the overall quality of evidence for this PICO question was very low.
Conclusions and research needs for this recommendation
The guideline panel suggests that during contingency or crisis situations involving shortages of N95 respirators reprocessed N95 respirators are to be reused instead of surgical masks as part of appropriate PPE when HCP conduct AGPs in patients with suspected or known COVID-19. Additional clinical studies are needed to characterize the risk of dispersal and acquisition of SARS-CoV-2 during AGPs in clinics, acute care and critical care wards and airborne infection isolation facilities.
Further investigations are needed to identify and validate optimal methods for reprocessing N95 respirators to meet safety requirements for HCP including infection rates associated with use of reprocessed N95 respirators.
Face shield or medical/surgical mask (extended use, reuse)
Recommendation 7: During contingency or crisis settings (respirator shortages), the IDSA guideline panel recommends that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or medical/surgical mask as a cover for the N95 respirator to allow for EXTENDED use as part of appropriate PPE*. (Strong recommendation, very low certainty evidence).
- Remark: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or medical/surgical mask covering the respirator.
Recommendation 8: During contingency or crisis settings (respirator shortages), the IDSA guideline panel suggests that healthcare personnel involved with aerosol-generating procedures on suspected or known COVID-19 patients add a face shield or medical/surgical mask as a cover for the N95 respirator to allow for REUSE as part of appropriate PPE*. (Conditional recommendation, very low certainty evidence).
- Remark: This recommendation assumes correct doffing sequence and hand hygiene are performed before and after removing the face shield or medical/surgical mask covering the respirator.
*Appropriate personal protective equipment includes—in addition to a mask or respirator—eye protection, gown, and gloves.
Summary of the evidence
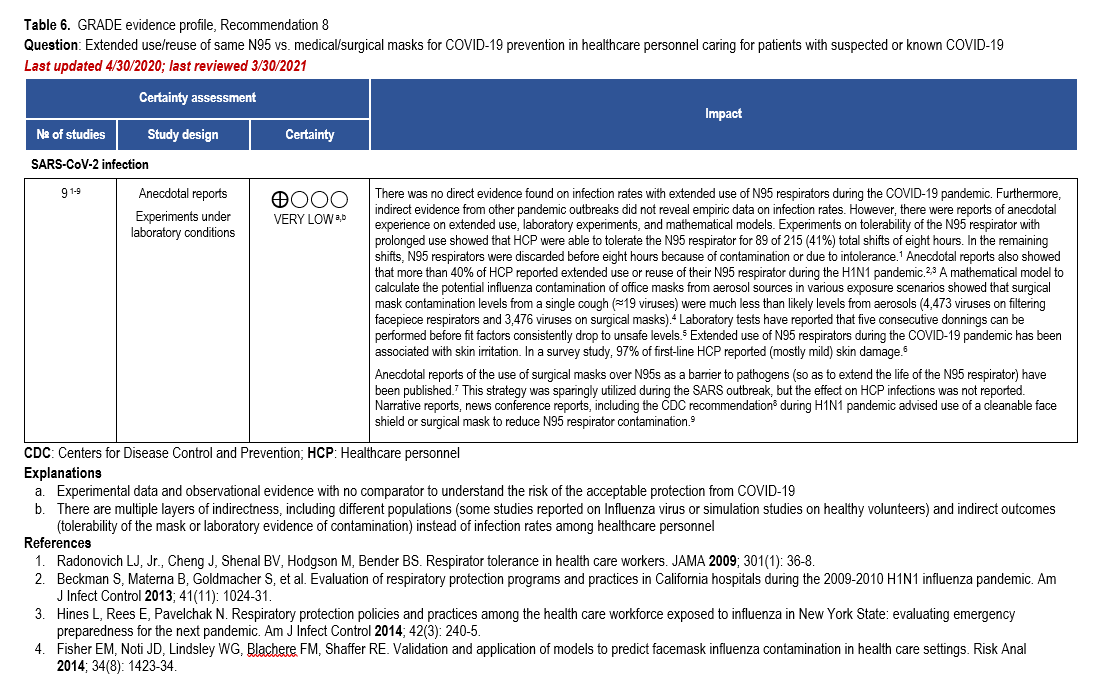
Extended use [67] is defined as wearing the same N95 respirator for multiple different and consecutive patient encounters without removal between encounters. The CDC recommends a maximum extended use period of 8-12 hours [67]. Reuse is defined as wearing the same N95 respirator for multiple different patient encounters but doffing between encounters. Unless the manufacturer specifies otherwise, the CDC suggests limiting N95 respirator reuse to no more than five times per device [67]. In contingency or crisis capacity settings (shortage of N95 respirators), no direct evidence on extended use or reuse of N95 respirator was identified. Additionally, no indirect comparative evidence on infection rates among HCP was identified (Table 6).
During the H1N1 pandemic, more than 40% of HCP reported extended use or reuse of an N95 respirator [68, 69]. During an influenza pandemic or other widespread respiratory pathogen outbreak, the CDC recommends the addition of a cleanable face shield on top of an N95 respirator to reduce respirator contamination [70]. Anecdotal reports of surgical masks being worn over N95 respirators were reported during the SARS outbreak. The face shield or surgical mask is felt to serve as a barrier to surface contamination thereby extending the life of the N95 respirator. However, the effect of extended use of this combination on infection rates among HCP has not been reported [71].
Based on laboratory evidence, in vitro testing on durability and endurance of N95 respirators suggests that three to five consecutive donnings can be performed before fit factors consistently drop predicting an unsafe fit [72]. In a survey of frontline HCP, 97% reported predominantly mild skin damage with extended use of an N95 respirator during the current COVID-19 pandemic [73].
Other considerations
The available evidence to inform this recommendation included anecdotal reports, experimental laboratory data and mathematical models. Strategies using a face shield or surgical mask to cover an N95 respirator and extend the life of the respirator were used in other pandemics. Additionally, in vitro testing was performed on durability and fit endurance of N95 respirators. The overall certainty of the evidence was low due to concerns about the observational data and lack of evidence on infection rates in HCP using N95 respirators for extended periods or reusing respirators.
Conclusions and research needs for this recommendation
The guideline panel recommends that, should extended or reuse of an N95 respirator become necessary in a contingency or crisis setting (i.e., N95 respirator shortage), HCP should place some type of barrier (face shield or surgical mask) over the N95 respirator while performing AGPs to reduce contamination of the N95 respirator. Either extended use or reuse strategies are preferred to a surgical mask alone when performing AGPs. These recommendations are based on indirect evidence suggesting that masks/respirators are frequently contaminated during AGPs and direct evidence suggesting that HCP routinely touch masks/respirators while wearing. As a result, the guideline panel believes these recommendations lead to increased safety for HCP and decreased risk of self-inoculation from a contaminated N95 respirator when worn for an extended period or reused on separate occasions.
Randomized controlled trials and prospective outcome registries are needed to inform strategies to prevent infection in HCP during contingency and crisis settings in which recommendations for use of PPE in conventional settings cannot be adhered to. Additional studies are also needed to characterize the true impact of extended use and reuse on N95 respirator fit and filtration, including identifying simple thresholds above which these strategies would no longer be recommended. Techniques for safely storing the N95 respirator between reuse (e.g., in a clean, breathable container) and preventing HCP contamination during donning and doffing require evaluation. Combining extended use or reuse with other conservation strategies such as alternating between different N95 respirators at a set interval or performing N95 disinfection may further improve safety and merits investigation.
Narrative Summaries
In addition to the clinical questions addressed above, the panel identified several infection prevention topics for which additional data are needed to formulate recommendations. Narrative summaries are provided below.
Does the use of N95 respirators require fit testing beyond ensuring a good seal?
N95 FFRS should be fit tested to demonstrate that a tight seal is maintained during routine activity. This will ensure maximal protection when HCP are involved in performing an AGP on COVID-19 patients [74]. Many different respirators are manufactured and variations in quality may exist [75, 76]; therefore, appropriate testing should occur to ensure appropriate fit. OSHA requires an initial fit test to identify the appropriate model, style, and size of the respirator [77]. Several methods for fit testing can be used to establish appropriate fit including inward leakage [78], qualitative fit test [79], quantitative eight-step fit test [80], fast five-step test [81], and, for certain respirators even a panel passing rate [82]. Visual or manual inspection of fit has been shown to be inaccurate and some form of quantitative fit testing provides the best method to ensure adequate fit and protection [83].
What is the role of PAPRs in contingency or crisis capacity settings?
Access to PAPRs may be even more limited due to cost and need for both routine maintenance and processes to ensure adequate cleaning and disinfection between uses; they can be considered if a healthcare facility has an established PAPR program that can appropriately service, clean, disinfect, and turn around these devices for the next user. Programs that incorporate PAPRs must also include programs for battery supply and maintenance. Healthcare personnel must complete formal competency training on how to use appropriately, as well as how to safely doff the PAPR hood to avoid self-contamination in the process [84]. Removal of PAPR hoods is more complicated than removal of an N95 or similar respirator and may increase the risk of self-contamination.
Should universal masking be used to prevent COVID-19 transmission in healthcare settings?
In addition to personal protection, the major purpose of universal masking is to limit transmission of viral particles from individuals wearing the mask (who may be asymptomatic or minimally symptomatic) to other individuals and the environment (i.e., source control).
Prior to COVID-19, we identified four studies of universal masking in a tertiary care hospital and certain high-risk settings. During the pandemic influenza A H1N1 in 2009, surgical mask use by HCP and visitors was promoted in the hospital. With these measures, only four (0.48%) of 836 persons who were exposed to laboratory-confirmed patients and staff with A/2009/H1N1 infection were confirmed to have A/2009/H1N1 infection. Not wearing a surgical mask, either by exposed persons during contact with index cases (4/4 vs. 264/832) or vice versa (4/4 vs. 300/832), was found to be a significant risk factor for the nosocomial acquisition of A/2009/H1N1 infection [85]. One prospective single-center study examined the impact of universal masking of all staff and visitors, regardless of symptoms or season, when in direct contact with hemopoietic stem cell transplant (HSCT) patients. Using a time series approach adjusted for season and year, the authors demonstrated a significant decrease in RVIs from 10.3% (95/920 patients) in the pre-mask period (2003-2009) to 4.4% (40/911) in the post-mask period (2010-2014), regardless of transplant type [86]. Another single-center quality improvement study assessed the impact of universal masking of all staff and visitors on the incidence of RVIs in a HSCT unit in a pre-post comparison without adjustment. There were 14 RVIs over 15,001 patient days in the pre-mask period versus two RVIs over of 15,608 patient days in the post-mask period [87]. A similar pre-post study of universal masking was performed in a neonatal intensive care unit (NICU) and special care nursery in Sweden. The incidence of RVIs during the pre-masking period (Jan 2014-Sept 2015) was compared to the incidence in the post-masking period (Oct 2015-Sept 2016). A non-significant decline in the RVI rate was observed in the special care nursery (5 per 10,000 patient days to 2 per 10,000), but declines were not observed in the NICU (1 per 10,000 vs. 2 per 10,000) [88].
Since the start of the COVID-19 pandemic, four studies have been published on the impact of universal masking in healthcare settings [89-92].
One study focused on the impact of universal masking of HCP and patients on the incidence of influenza A, influenza B and RSV in current and corresponding seasons in the past three years [89]. After implementation of universal masking in the healthcare setting, all nosocomial RVIs were eliminated, demonstrating the importance of masks in interrupting transmission of respiratory viruses [89]. Three additional U.S.-based studies compared COVID-19 incidence rates among HCP over time (pre-intervention, intervention, and post-intervention periods). Similar findings were noted across studies with significant declines in positivity after implementation of universal masking of HCP, with or without universal masking of patients [90-92]. Two studies also compared similar trends in community incidence rates during the same period [91, 92], and one also estimated differences between healthcare-associated and community-associated HCP infection rates [91], lending further support for the use of universal masking to prevent COVID-19 transmission in healthcare settings.
These clinical studies likely demonstrated declines to which concomitant adherence to hand hygiene, maintaining physical distancing, and other appropriate precautions in high-risk settings likely contributed. Nonetheless, there is likely significant benefit associated with universal masking in the absence of resource constraints.
Does the addition of a “negative pressure room” / airborne infection isolation room provide increased safety for healthcare personnel working with suspected or known COVID-19 patients?
The role of an airborne infection isolation room (AIIR), or negative pressure room, as an intervention to increase safety for HCP caring for suspected or known COVID-19 patients remains unclear. Negative pressure rooms are routinely used to prevent transmission of pathogens spread over long distances by small airborne particles nuclei, such as measles virus, Mycobacterium tuberculosis, or varicella-zoster virus. To date, there are no concrete data to suggest that SARS-CoV-2 is routinely spread via long-distance transmission in acute care hospitals, but this is a rapidly evolving area of research [93, 94]. Additional studies are required to better characterize transmission dynamics of SARS-CoV-2 and emerging variants and to tailor effective infection prevention strategies to protect HCP. When availability of AIIR is limited, facilities may consider reserving these for patients undergoing AGPs. Healthcare facilities should ensure adequate levels of ventilation and filtration in all areas where care for patients with known or suspected COVID occurs [95].
Discussion
Due to limited RCTs, many infection prevention and control (IPC) recommendations are based on observational data, quality improvement projects, and indirect evidence. This is despite the large impact of healthcare-associated infections (HAIs), estimated to lead to 1.7 million infections and nearly 100,000 deaths per year in the United States [96]. Most IPC programs focus on a variety of high-risk HAIs, like central-line associated blood stream infections and Clostridioides difficile infection, that can be monitored and are amenable to targeted interventions. Intervening to prevent these infections also may prevent HAIs that are not part of surveillance programs by improving overall hand hygiene, environmental cleaning, and other behaviors [97]. These programs are also responsible for recommendations for PPE to protect HCP and to respond to infectious disease outbreaks. However, the lack of evidence -based recommendations continues to be a significant challenge when responding to outbreaks that put HCP at risk. This challenge is magnified when faced with a novel pathogen, like SARS-CoV-2, that has spread rapidly across the globe and is associated with a very broad spectrum of symptoms and transmission risk. With rapidly moving and large epidemics and inadequate preparation for pandemics, the supply of PPE is put at risk, further complicating how recommendations for PPE usage are developed and implemented [98].
The SARS-CoV-2 pandemic was first described as an outbreak of pneumonia at the end of December 2019 [99]. The rapid identification of the virus and its relation to human and zoonotic coronaviruses led to early recommendations and expectations for modes of transmission [100, 101]. Over the following weeks, hospital and clinic-based IPC recommendations were proposed that evolved in response to reports from the field and laboratory. A significant challenge has been the reliance on clinical observations, molecular assays as proxies for transmission, extrapolations from SARS-CoV, MERS-CoV, human coronaviruses and influenza, and other indirect evidence. This is problematic as we learn more about SARS-CoV-2 and its pathogenesis, such as the rapid decrease in viral burden following the onset of symptoms, which is, for example, different than the kinetics of SARS-CoV [23, 102].
The WHO published guidelines for the use of PPE that have been mirrored by the national public health authorities in Canada [103], Australia [104], and England [105]. The CDC adopted more conservative recommendations early in the pandemic and has maintained these recommendations, including the use of an N95 respirator for the routine care of patients with known or suspected COVID-19. Although earlier CDC guidance allowed for the use of medical masks for this level of care when supplies were low, a more recent update has listed mitigation strategies that prioritize respirator use (including reuse) [106]. These conflicting guidelines have put IPC/environmental health (EH) leaders, IPC/EH teams, and HCP in challenging positions, especially when balancing ongoing supply chain challenges and an unclear forecast for the trajectory of the epidemic. One agreed upon objective is to maintain HCP safety in all patient care scenarios while balancing patient safety and access to care. When supply chains are exhausted, the only option is to retreat to maximal PPE coverage of the HCP and to not remove PPE between patient encounters (extended use). This, combined with breakdowns in administrative and engineering controls, can lead to transmission of pathogens within the healthcare setting.
To establish a baseline, not a ceiling, for IPC recommendations and to highlight opportunities for research, the IDSA guideline panel used the best available evidence to provide recommendations on the use of PPE and potential risks with the healthcare environment. We recognize there are many more questions that desperately need answers, and we hope to be able to pursue additional questions in the near future. The panel recognizes the rapidly evolving nature of the pandemic, the incredible pace of discovery and growing access to clinical and laboratory data. Recommendations were made where possible based on the evidence. In the absence of even indirect data, recommendations were made that prioritized the safety of HCP. The panel hopes that highlighting the distinct lack of data to guide many recommendations will increase the attention of the research community and funders to address these gaps. Answers will likely require observational and non-experimental studies, but the panel would like to emphasize that RCTs are possible and should be encouraged.
HCP rely on PPE for their safety and the data to support specific pieces or combinations of equipment are critical. Studies must include the other elements of PPE and practices, including the use of hand hygiene, since no PPE is used in isolation. The practice of hand hygiene when using PPE is critical to prevent contamination and transmission [107, 108], but adherence to this practice in studies of PPE use is not routinely reported. The role of education, HCP comfort and familiarity with equipment, and PPE use in routine clinical scenarios (e.g., resuscitation, psychiatric emergency department, or by public safety officers) also need attention. Other tools that can increase adherence and safety, such as the use of trained observer for donning and doffing, are being used heterogeneously and without guidance in most regions. The role of SARS-CoV-2 immunity in HCP, either due to prior infection or, more commonly, due to vaccination, has also not been addressed in relation to PPE recommendations.
Unlike areas of clinical research that include treatment and diagnostics, the use of PPE and HCP practices are embedded in human behavior, human factors, and environmental conditions. The participation of researchers with expertise in human behavior, ergonomics, psychology, physics, aerosol science, building design, and anthropology are needed to evaluate how and why PPE is used by HCP, perceptions of risk, and adherence to local guidelines [109]. While not always feasible, the panel recommends that outcome measures be used in research studies of PPE and, if not possible, rigorous definitions of process measures that incorporate other measures of HCP behavior to ensure comparability of studies (e.g., hand hygiene). More data also is needed on the types of procedures that are considered higher risk for transmission (i.e., AGPs) as well as considerations for PPE and AIIR or negative pressure rooms for HCP involved with AGPs.
Healthcare systems and smaller clinical settings, like clinics attached to shelters and in other congregate settings, are currently being called upon to protect their healthcare workforce in the face of a pandemic. This situation has an impact on HCP directly in their role as essential personnel leaving their homes to work and as members of communities. The risk of SARS-CoV-2 acquisition exists in both scenarios, which means that non-immune HCP will continue to become infected even with appropriate use of PPE within the patient care environment. This highlights the important role of vaccination for HCP in addition to appropriate PPE. COVID-19 vaccines have clearly demonstrated a reduction in infection, risk of hospitalization, and mortality, and should be required for all HCP, for their own safety as well as the safety of their co-workers and patients [110, 111]. Finally, while determining how to address the research gaps for PPE and protocols for SARS-CoV-2, the panel also recommends attention to how PPE use is perceived by patients and what tools are needed to mediate communication between HCP and specific patient communities, such as people who use lip reading or depend on facial recognition. These questions and many more remain in critical need of attention.
This is a living guideline that will be frequently updated as new data emerges. Updates and changes to the guideline will be posted to the IDSA website.
Notes
Acknowledgement
The expert panel thanks the Infectious Diseases Society of America for supporting guideline development, and specifically Cynthia Sears, Dana Wollins, Genet Demisashi, and Rebecca Goldwater for their continued support throughout the guideline process.
Financial Support
This project was funded by IDSA.
COI Summary
The following list displays what has been reported to IDSA. To provide thorough transparency, IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential COIs is determined by a review process that includes assessment by the Board of Directors liaison to the Standards and Practice Guideline Committee and, if necessary, the COI and Ethics Committee. The assessment of disclosed relationships for possible COI is based on the relative weight of the financial relationship (i.e., monetary amount) and the relevance of the relationship (i.e., the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. P.D. receives research funding from University Hospitals Seidman Cancer Center. D.A. receives research funding from the CDC, Agency for Healthcare Research and Quality (AHRQ) and National Institutes of Health (NIH); and receives Duke University Educational Program funding from the National Football League. V.C. receives research funding from the Health and Medical Research Fund. J.G.C. serves as an advisor in pediatric infectious diseases and infection for Children’s Health Alliance and in infectious diseases/infection control for Oregon Health Authority; and receives research funding from the CDC. A.D. receives research funding from the National Heart, Lung, and Blood Institute, NIH, A-Lung and Sunnybrook Health Sciences Centre. M.J. serves as a consultant for Gilead Sciences, Inc. and receives research funding from Gilead Sciences, Inc., Regeneron Pharmaceuticals, Inc., Janssen Pharmaceuticals, Merck, GlaxoSmithKline, ViiV Health care, and VasoGene. G.L. serves as vice chair for the Advisory Committee on Immunization Practices. S.L. receives research funding from the CDC, Barnes-Jewish Hospital Foundation, and ContraFect Corporation. A.M. receives research funding from Pfizer, Inc., Merck, Sanofi Pasteur, Seqirus, the Canadian Institutes of Health Research, the Joint Programming Initiative on Antimicrobial Resistance, the Canadian Immunization Research Network, the Ontario Ministry of Health, and the Natural Sciences and Engineering Research Council; receives funding from Build in Canada Innovation Program Funding Program; serves as an advisory board member for GlaxoSmithKline, Merck, Cidara and Seqirus; and receives honoraria for lectures on influenza for Cidara. M.H.M. receives research funding from AHRQ, the Endocrine Society, the Society for Vascular Surgery, and The American Society of Hematology; and is a Board member for the Evidence Foundation. Y.F.Y. receives honoraria for evidence reviews and teaching from the Evidence Foundation and for evidence reviews for the American Gastroenterological Association; and serves as a Director for the Evidence Foundation and the U.S. GRADE Network. All other authors: no disclosures reported. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
IDSA Disclaimer
It is important to realize that guidelines cannot always account for individual variation among patients. They are assessments of current scientific and clinical information provided as an educational service; are not continually updated and may not reflect the most recent evidence (new evidence may emerge between the time information is developed and when it is published or read); should not be considered inclusive of all proper treatments methods of care, or as a statement of the standard of care; do not mandate any particular course of medical care; and are not intended to supplant physician judgment with respect to particular patients or special clinical situations. Whether and the extent to which to follow guidelines is voluntary, with the ultimate determination regarding their application to be made by the physician in the light of each patient’s individual circumstances. While IDSA makes every effort to present accurate, complete, and reliable information, these guidelines are presented “as is” without any warranty, either express or implied. IDSA (and its officers, directors, members, employees, and agents) assume no responsibility for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with these guidelines or reliance on the information presented.
The guidelines represent the proprietary and copyrighted property of IDSA. Copyright 2020 Infectious Diseases Society of America. All rights reserved. No part of these guidelines may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of IDSA. Permission is granted to physicians and health care providers solely to copy and use the guidelines in their professional practices and clinical decision-making. No license or permission is granted to any person or entity, and prior written authorization by IDSA is required, to sell, distribute, or modify the guidelines, or to make derivative works of or incorporate the guidelines into any product, including but not limited to clinical decision support software or any other software product. Except for the permission granted above, any person or entity desiring to use the guidelines in any way must contact IDSA for approval in accordance with the terms and conditions of third-party use, in particular any use of the guidelines in any software product.
References
Committee on Guidance for Establishing Crisis Standards of Care for Use in Disaster Situations, Institute of Medicine. Crisis Standards of Care: A Systems Framework for Catastrophic Disaster Response. Washington, DC: National Academies Press, 2012.
- Hick JL, Barbera JA, Kelen GD. Refining surge capacity: conventional, contingency, and crisis capacity. Disaster Med Public Health Prep 2009; 3(2 Suppl): S59-67.
- Centers for Disease Control and Prevention. Strategies for Optimizing the Supply of Facemasks. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html. Accessed 23 April.
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020; 382(18): 1708-20.
- World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 75. Geneva: World Health Organization, 2020 4 April.
- Al Mutair A, Al Mutairi A, Alhumaid S, et al. Examining and investigating the impact of demographic characteristics and chronic diseases on mortality of COVID-19: Retrospective study. PLoS One 2021; 16(9): e0257131.
- Nuno M, Garcia Y, Rajasekar G, Pinheiro D, Schmidt AJ. COVID-19 hospitalizations in five California hospitals: a retrospective cohort study. BMC Infect Dis 2021; 21(1): 938.
- Banoei MM, Dinparastisaleh R, Zadeh AV, Mirsaeidi M. Machine-learning-based COVID-19 mortality prediction model and identification of patients at low and high risk of dying. Crit Care 2021; 25(1): 328.
- Riera J, Roncon-Albuquerque R, Jr., Fuset MP, Alcantara S, Blanco-Schweizer P, Group ES. Increased mortality in patients with COVID-19 receiving extracorporeal respiratory support during the second wave of the pandemic. Intensive Care Med 2021.
- Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One 2020; 15(7): e0235458.
- Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020; 323(11): 1061-9.
- Centers for Disease Control and Prevention. Hospitalization and Death by Race/Ethnicity. Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html. Accessed 16 June 2021.
- Zambrano LD, Ellington S, Strid P, et al. Update: Characteristics of Symptomatic Women of Reproductive Age with Laboratory-Confirmed SARS-CoV-2 Infection by Pregnancy Status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(44): 1641-7.
- Jones TC, Biele G, Muhlemann B, et al. Estimating infectiousness throughout SARS-CoV-2 infection course. Science 2021; 373(6551).
- Huo X, Chen J, Ruan S. Estimating asymptomatic, undetected and total cases for the COVID-19 outbreak in Wuhan: a mathematical modeling study. BMC Infect Dis 2021; 21(1): 476.
- Chen W, Hu Z, Yi C, et al. An unusual COVID-19 case with over four months of viral shedding in the presence of low neutralizing antibodies: a case report. J Biomed Res 2020; 34(6): 470-4.
- Widders A, Broom A, Broom J. SARS-CoV-2: The viral shedding vs infectivity dilemma. Infect Dis Health 2020; 25(3): 210-5.
- Mancuso P, Venturelli F, Vicentini M, et al. Temporal profile and determinants of viral shedding and of viral clearance confirmation on nasopharyngeal swabs from SARS-CoV-2-positive subjects: a population-based prospective cohort study in Reggio Emilia, Italy. BMJ Open 2020; 10(8): e040380.
- Greenhalgh T, Jimenez JL, Prather KA, Tufekci Z, Fisman D, Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021; 397(10285): 1603-5.
- Morawska L, Milton DK. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin Infect Dis 2020; 71(9): 2311-3.
- Sehulster L, Chinn RY, Cdc, Hicpac. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC) (Appendix B: Air). MMWR Recomm Rep 2003; 52(RR-10): 1-42.
- Cheng HY, Jian SW, Liu DP, et al. Contact Tracing Assessment of COVID-19 Transmission Dynamics in Taiwan and Risk at Different Exposure Periods Before and After Symptom Onset. JAMA Intern Med 2020; 180(9): 1156-63.
- McEllistrem MC, Clancy CJ, Buehrle DJ, et al. SARS-CoV-2 is associated with high viral loads in asymptomatic and recently symptomatic healthcare workers. PLoS One 2021; 16(3): e0248347.
- Morgan RL, Florez I, Falavigna M, et al. Development of rapid guidelines: 3. GIN-McMaster Guideline Development Checklist extension for rapid recommendations. Health Res Policy Syst 2018; 16(1): 63.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64(4): 395-400.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2 ed. Chichester (UK): John Wiley & Sons, 2019.
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011; 64(4): 383-94.
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available at: https://gradepro.org/.
- Andrews JC, Schunemann HJ, Oxman AD, et al. GRADE guidelines: 15. Going from evidence to recommendation-determinants of a recommendation's direction and strength. J Clin Epidemiol 2013; 66(7): 726-35.
- Occupational Safety and Health Administration, U.S. Department of Labor. Respiratory Infection Control: Respirators Versus Surgical Masks. In: OSHA Fact Sheet, Occupational Safety and Health Administration, 2009.
- Sims MD, Maine GN, Childers KL, et al. Coronavirus Disease 2019 (COVID-19) Seropositivity and Asymptomatic Rates in Healthcare Workers Are Associated with Job Function and Masking. Clin Infect Dis 2021; 73(Suppl 2): S154-S62.
- Akinbami LJ, Vuong N, Petersen LR, et al. SARS-CoV-2 Seroprevalence among Healthcare, First Response, and Public Safety Personnel, Detroit Metropolitan Area, Michigan, USA, May-June 2020. Emerg Infect Dis 2020; 26(12): 2863-71.
- Chen Y, Tong X, Wang J, et al. High SARS-CoV-2 antibody prevalence among healthcare workers exposed to COVID-19 patients. J Infect 2020; 81(3): 420-6.
- Piapan L, De Michieli P, Ronchese F, et al. COVID-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy. J Hosp Infect 2020; 106(3): 626-8.
- Murakami E, Ghatak-Roy A, Popova M, et al. COVID-19 infection among emergency department healthcare providers in a large tertiary academic medical center following the peak of the pandemic. Am J Emerg Med 2021; 40: 27-31.
- Schmitz D, Vos M, Stolmeijer R, et al. Association between personal protective equipment and SARS-CoV-2 infection risk in emergency department healthcare workers. Eur J Emerg Med 2021; 28(3): 202-9.
- Khalil MM, Alam MM, Arefin MK, et al. Role of Personal Protective Measures in Prevention of COVID-19 Spread Among Physicians in Bangladesh: a Multicenter Cross-Sectional Comparative Study. SN Compr Clin Med 2020: 1-7.
- Chatterjee P, Anand T, Singh KJ, et al. Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19. Indian J Med Res 2020; 151(5): 459-67.
- Kohler PP, Kahlert CR, Sumer J, et al. Prevalence of SARS-CoV-2 antibodies among Swiss hospital workers: Results of a prospective cohort study. Infect Control Hosp Epidemiol 2021; 42(5): 604-8.
- Wang X, Pan Z, Cheng Z. Association between 2019-nCoV transmission and N95 respirator use. J Hosp Infect 2020; 105(1): 104-5.
- Ambrosch A, Rockmann F, Klawonn F, Lampl B. Effect of a strict hygiene bundle for the prevention of nosocomial transmission of SARS-CoV-2 in the hospital: a practical approach from the field. J Infect Public Health 2020; 13(12): 1862-7.
- Bhaskar ME, Arun S. SARS-CoV-2 Infection Among Community Health Workers in India Before and After Use of Face Shields. JAMA 2020; 324(13): 1348-9.
- Ong SWX, Tan YK, Sutjipto S, et al. Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Infect Control Hosp Epidemiol 2020: 1-3.
- Casanova LM, Rutala WA, Weber DJ, Sobsey MD. Effect of single- versus double-gloving on virus transfer to health care workers' skin and clothing during removal of personal protective equipment. Am J Infect Control 2012; 40(4): 369-74.
- Centers for Disease Control and Prevention. Sequence for Personal Protective Equipment (PPE). n.d.
- Verbeek JH, Rajamaki B, Ijaz S, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev 2020; 5: CD011621.
- Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012; 7(4): e35797.
- van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med 2020; 382(16): 1564-7.
- Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA 2020.
- Nguyen LH, Drew DA, Graham MS, et al. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health 2020; 5(9): e475-e83.
- Fischer RJ, Morris DH, van Doremalen N, et al. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg Infect Dis 2020; 26(9).
- Levine C, Grady C, Block T, et al. Use, re-use or discard? Quantitatively defined variance in the functional integrity of N95 respirators following vaporized hydrogen peroxide decontamination during the COVID-19 pandemic. J Hosp Infect 2021; 107: 50-6.
- Lieu A, Mah J, Zanichelli V, Exantus RC, Longtin Y. Impact of extended use and decontamination with vaporized hydrogen peroxide on N95 respirator fit. Am J Infect Control 2020; 48(12): 1457-61.
- Maranhao B, Scott AW, Scott AR, et al. Probability of fit failure with reuse of N95 mask respirators. Br J Anaesth 2020; 125(3): e322-e4.
- Ibanez-Cervantes G, Bravata-Alcantara JC, Najera-Cortes AS, et al. Disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria using hydrogen peroxide plasma: Impact on the reutilization of disposable devices. Am J Infect Control 2020; 48(9): 1037-41.
- Cramer AK, Plana D, Yang H, et al. Analysis of SteraMist ionized hydrogen peroxide technology in the sterilization of N95 respirators and other PPE. Sci Rep 2021; 11(1): 2051.
- Ozog DM, Sexton JZ, Narla S, et al. The effect of ultraviolet C radiation against different N95 respirators inoculated with SARS-CoV-2. Int J Infect Dis 2020; 100: 224-9.
- Aljabo A, Mueller E, Abdul-Azeez D, Hoare T, Jain A. Gravity steam reprocessing in healthcare facilities for the reuse of N95 respirators. J Hosp Infect 2020; 106(4): 698-708.
- Carrillo IO, Floyd ACE, Valverde CM, Tingle TN, Zabaneh FR. Immediate-use steam sterilization sterilizes N95 masks without mask damage. Infect Control Hosp Epidemiol 2020; 41(9): 1104-5.
- Zulauf KE, Green AB, Nguyen Ba AN, et al. Microwave-Generated Steam Decontamination of N95 Respirators Utilizing Universally Accessible Materials. mBio 2020; 11(3).
- Daeschler SC, Manson N, Joachim K, et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. CMAJ 2020; 192(41): E1189-E97.
- Anderegg L, Meisenhelder C, Ngooi CO, et al. A scalable method of applying heat and humidity for decontamination of N95 respirators during the COVID-19 crisis. PLoS One 2020; 15(7): e0234851.
- Czubryt MP, Stecy T, Popke E, et al. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J Hosp Infect 2020; 106(2): 277-82.
- Oh C, Araud E, Puthussery JV, et al. Dry heat as a decontamination method for N95 respirator reuse. Environmental Science & Technology Letters 2020; 7(9): 677-82.
- Centers for Disease Control and Prevention. Strategies for Optimizing the Supply of N95 Respirators. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/respirators-strategy/index.html. Accessed 16 April.
- Beckman S, Materna B, Goldmacher S, et al. Evaluation of respiratory protection programs and practices in California hospitals during the 2009-2010 H1N1 influenza pandemic. Am J Infect Control 2013; 41(11): 1024-31.
- Hines L, Rees E, Pavelchak N. Respiratory protection policies and practices among the health care workforce exposed to influenza in New York State: evaluating emergency preparedness for the next pandemic. Am J Infect Control 2014; 42(3): 240-5.
- Centers for Disease Control and Prevention. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. Available at: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html. Accessed 16 April 2020.
- Roberge RJ. Effect of surgical masks worn concurrently over N95 filtering facepiece respirators: extended service life versus increased user burden. J Public Health Manag Pract 2008; 14(2): E19-26.
- Bergman MS, Viscusi DJ, Zhuang Z, Palmiero AJ, Powell JB, Shaffer RE. Impact of multiple consecutive donnings on filtering facepiece respirator fit. Am J Infect Control 2012; 40(4): 375-80.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. J Am Acad Dermatol 2020; 82(5): 1215-6.
- Reponen T, Lee SA, Grinshpun SA, Johnson E, McKay R. Effect of fit testing on the protection offered by n95 filtering facepiece respirators against fine particles in a laboratory setting. Ann Occup Hyg 2011; 55(3): 264-71.
- Lawrence RB, Duling MG, Calvert CA, Coffey CC. Comparison of performance of three different types of respiratory protection devices. J Occup Environ Hyg 2006; 3(9): 465-74.
- Delaloye JR, Vernez D, Suarez G, et al. Distribution of low quality filtering facepiece respirators during the COVID-19 pandemic: an independent analysis of the situation in Switzerland. Swiss Med Wkly 2021; 151: w20459.
- D'Alessandro MM, Casey M, Cichowicz JK, Centers for Disease Control and Prevention. The Need for Fit Testing During Emerging Infectious Disease Outbreaks. Available at: https://blogs.cdc.gov/niosh-science-blog/2020/04/01/fit-testing-during-outbreaks/. Accessed 16 April 2020.
- Zhuang Z, Bergman M, Brochu E, et al. Temporal changes in filtering-facepiece respirator fit. J Occup Environ Hyg 2016; 13(4): 265-74.
- Lin YC, Chen CP. Characterization of small-to-medium head-and-face dimensions for developing respirator fit test panels and evaluating fit of filtering facepiece respirators with different faceseal design. PLoS One 2017; 12(11): e0188638.
- Hauge J, Roe M, Brosseau LM, Colton C. Real-time fit of a respirator during simulated health care tasks. J Occup Environ Hyg 2012; 9(10): 563-71.
- Sietsema M, Brosseau LM. Are quantitative fit factors predictive of respirator fit during simulated healthcare activities? J Occup Environ Hyg 2018; 15(12): 803-9.
- Zhuang Z, Bergman M, Lei Z, Niezgoda G, Shaffer R. Recommended test methods and pass/fail criteria for a respirator fit capability test of half-mask air-purifying respirators. J Occup Environ Hyg 2017; 14(6): 473-81.
- O'Kelly E, Arora A, Pirog S, Ward J, Clarkson PJ. Comparing the fit of N95, KN95, surgical, and cloth face masks and assessing the accuracy of fit checking. PLoS One 2021; 16(1): e0245688.
- Tompkins BM, Kerchberger JP. Special article: personal protective equipment for care of pandemic influenza patients: a training workshop for the powered air purifying respirator. Anesth Analg 2010; 111(4): 933-45.
- Cheng VC, Tai JW, Wong LM, et al. Prevention of nosocomial transmission of swine-origin pandemic influenza virus A/H1N1 by infection control bundle. J Hosp Infect 2010; 74(3): 271-7.
- Sung AD, Sung JAM, Thomas S, et al. Universal Mask Usage for Reduction of Respiratory Viral Infections After Stem Cell Transplant: A Prospective Trial. Clin Infect Dis 2016; 63(8): 999-1006.
- Sokol KA, De la Vega-Diaz I, Edmondson-Martin K, et al. Masks for prevention of respiratory viruses on the BMT unit: results of a quality initiative. Transpl Infect Dis 2016; 18(6): 965-7.
- Yeo KT, Yung CF, Chiew LC, et al. Universal Mask Policy in the Neonatal Unit to Reduce Respiratory Viral Infections. Clin Infect Dis 2017; 64(6): 817.
- Wong SC, Lam GK, AuYeung CH, et al. Absence of nosocomial influenza and respiratory syncytial virus infection in the coronavirus disease 2019 (COVID-19) era: Implication of universal masking in hospitals. Infect Control Hosp Epidemiol 2021; 42(2): 218-21.
- Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association Between Universal Masking in a Health Care System and SARS-CoV-2 Positivity Among Health Care Workers. JAMA 2020; 324(7): 703-4.
- Seidelman JL, Lewis SS, Advani SD, et al. Universal masking is an effective strategy to flatten the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) healthcare worker epidemiologic curve. Infect Control Hosp Epidemiol 2020; 41(12): 1466-7.
- Lan FY, Christophi CA, Buley J, et al. Effects of universal masking on Massachusetts healthcare workers' COVID-19 incidence. Occup Med (Lond) 2020; 70(8): 606-9.
- Thompson HA, Mousa A, Dighe A, et al. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Setting-specific Transmission Rates: A Systematic Review and Meta-analysis. Clin Infect Dis 2021; 73(3): e754-e64.
- Billah MA, Miah MM, Khan MN. Reproductive number of coronavirus: A systematic review and meta-analysis based on global level evidence. PLoS One 2020; 15(11): e0242128.
- Gettings J, Czarnik M, Morris E, et al. Mask Use and Ventilation Improvements to Reduce COVID-19 Incidence in Elementary Schools - Georgia, November 16-December 11, 2020. MMWR Morb Mortal Wkly Rep 2021; 70(21): 779-84.
- Klevens RM, Edwards JR, Richards CL, Jr., et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep 2007; 122(2): 160-6.
- Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med 2011; 364(15): 1419-30.
- He S, Bala R, Anupindi R, Ranney ML. Effective supply chain surveillance for PPE. Lancet 2021; 397(10286): 1706-7.
- World Health Organization. Pneumonia of unknown cause–China. Available at: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/.
- Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020; 579(7798): 265-9.
- Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med 2020; 382(8): 727-33.
- He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med 2020.
- Government of Canada. COVID-19 medical masks and respirators: Overview. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/medical-devices/personal-protective-equipment/medical-masks-respirators/overview.html. Accessed 16 June 2021.
- Australian Government, Department of Health, Infection Control Expert Group. Guidance on the use of personal protective equipment (PPE) for health care workers in the context of COVID-19. Sydney, New South Wales, Australia: Australian Commission on Safety and Quality in Health Care, 2021 10 June.
- UK Health Security Agency. COVID-19: infection prevention and control (IPC). United Kingdom: Department of Health and Social Care, 2021 29 September.
- Centers for Disease Control and Prevention. Interim Infection Prevention and Control Recommendations for Healthcare Personnel During the Coronavirus Disease 2019 (COVID-19) Pandemic. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html. Accessed 16 June 2021.
- Mumma JM, Durso FT, Ferguson AN, et al. Human Factors Risk Analyses of a Doffing Protocol for Ebola-Level Personal Protective Equipment: Mapping Errors to Contamination. Clin Infect Dis 2018; 66(6): 950-8.
- Tomas ME, Kundrapu S, Thota P, et al. Contamination of Health Care Personnel During Removal of Personal Protective Equipment. JAMA Intern Med 2015; 175(12): 1904-10.
- Gurses AP, Dietz AS, Nowakowski E, et al. Human factors-based risk analysis to improve the safety of doffing enhanced personal protective equipment. Infect Control Hosp Epidemiol 2019; 40(2): 178-86.
- Shemtob L, Ferris M, Asanati K, Majeed A. Vaccinating healthcare workers against covid-19. BMJ 2021; 374: n1975.
- Waldman SE, Adams JY, Albertson TE, et al. Real-world impact of vaccination on coronavirus disease 2019 (COVID-19) incidence in healthcare personnel at an academic medical center. Infect Control Hosp Epidemiol 2021: 1-7.
Supplementary Information
Additional Resources
Donning and Doffing Videos
- Society for Healthcare Epidemiology of America
- Rush University Medical Center
- University of Texas Southwestern Medical Center
- Queen Mary Hospital - Hong Kong West Cluster