COVID-19 Guideline, Part 1: Treatment and Management
COVID-19 Guideline, Part 2: Infection Prevention
COVID-19 Guideline, Part 3: Molecular Testing
COVID-19 Guideline, Part 5: Antigen Testing
Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians
Update History
August 18, 2020
Version 1.0 of the guideline has been released.
Abstract
Background: The role of serologic testing for SARS-CoV-2 has evolved during the pandemic as seroprevalence in global populations has increased. The Infectious Diseases Society of America (IDSA) convened an expert panel to perform a systematic review of the coronavirus disease 2019 (COVID-19) serology literature and construct updated best practice guidance related to SARS-CoV-2 serologic testing. This guideline is an update to the fourth in a series of rapid, frequently updated COVID-19 guidelines developed by IDSA.
Objective: To develop evidence-based recommendations and identify unmet research needs pertaining to the use of anti-SARS-CoV-2 antibody tests for diagnosis, decisions related to vaccination and administration of monoclonal antibodies or convalescent plasma in immunocompromised patients, and identification of a serologic correlate of immunity.
Methods: A multidisciplinary panel of infectious diseases clinicians, clinical microbiologists and experts in systematic literature reviewed, identified, and prioritized clinical questions related to the use of SARS-CoV-2 serologic tests. Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to assess the certainty of evidence and make testing recommendations.
Results: The panel recommends against serologic testing to diagnose SARS-CoV-2 infection in the first two weeks after symptom onset (strong recommendations, low certainty of evidence). Serologic testing should not be used to provide evidence of COVID-19 in symptomatic patients with a high clinical suspicion and repeatedly negative nucleic acid amplification test results (strong recommendation, very low certainty of evidence). Serologic testing may assist with the diagnosis of multisystem inflammatory syndrome in children (strong recommendation, very low certainty of evidence). To seek evidence for prior SARS-CoV-2 infection, the panel suggests testing for IgG, IgG/IgM, or total antibodies to nucleocapsid protein three to five weeks after symptom onset (conditional recommendation, low certainty of evidence). In individuals with previous SARS-CoV-2 infection or vaccination, we suggest against routine serologic testing given no demonstrated benefit to improving patient outcomes (conditional recommendation, very low certainty of evidence.) The panel acknowledges further that a negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy.
Conclusions: The high seroprevalence of antibodies against SARS-CoV-2 worldwide limits the utility of detecting anti-SARS CoV-2 antibody. The certainty of available evidence supporting the use of serology for diagnosis was graded as very low to low. Future studies should use serologic assays calibrated to a common reference standard.
Executive Summary
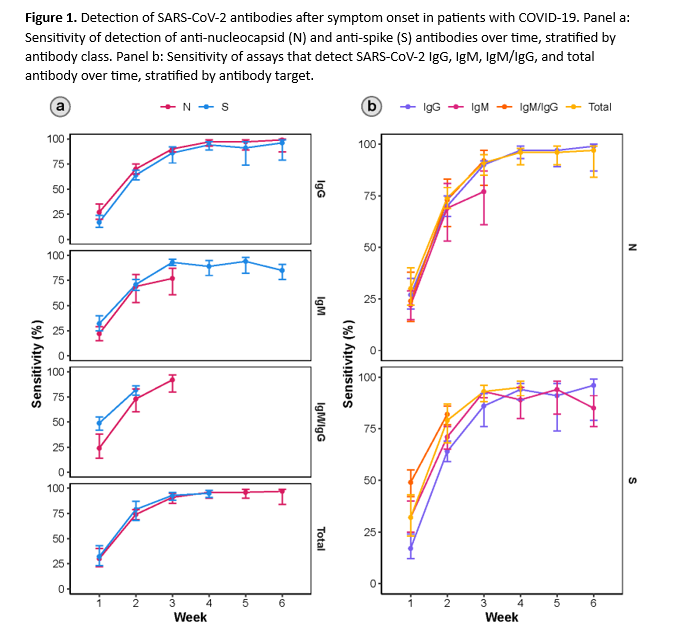
As of December 2022, more than 96% of the U.S. population was estimated to have antibodies against SARS-CoV-2 [1]. While global seroprevalence data are more sparse, similar estimates have been reported from numerous other countries and regions [2]. The utility of anti-SARS-CoV-2 antibody testing for diagnostic or epidemiologic purposes has declined as global seroprevalence due to vaccination and natural infection has increased. The current IDSA Guideline for Serologic Testing in the Diagnosis of SARS-COV-2 Infection reflects this evolution. The panel now makes a strong (versus weak) recommendation against serologic testing to diagnose acute COVID-19 in the first 2 weeks after symptom onset. Similarly, the updated guideline recommends strongly against (versus previously recommending weakly for) using IgG antibodies to provide evidence of COVID-19 in symptomatic patients with a high clinical suspicion and repeatedly negative nucleic acid amplification tests (NAAT). Unlike NAAT which detect viral RNA, antibody-based assays measure the host’s humoral immune response to current or past infection. Anti-SARS-CoV-2 antibodies typically become detectable more than two weeks after onset of symptoms (Figure 1). As a result, SARS-CoV-2 serology lacks sufficient sensitivity to confidently exclude a diagnosis of coronavirus disease 2019 (COVID-19) when antibodies are not detected in the acute phase of illness. Furthermore, given the very high baseline seroprevalence of anti-SARS-CoV-2 antibodies in most populations, a positive serologic result may likely be due to vaccination, or a distant history of COVID-19 as opposed to an acute or recent SARS-CoV-2 infection.
For the current update, the panel identified very few clinical scenarios in which serologic testing may be useful. The first to assist in the diagnosis of Multisystem Inflammatory Syndrome in children (MIS-C). In addition, the panel agreed that a negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy, such as convalescent plasma or monoclonal antibodies, if such therapy were available, or to prioritize administration of monoclonal therapies when supplies are limited. When antibody testing is done, the panel makes a weak recommendation for using SARS-CoV-2 IgG, IgG/IgM, or total antibodies and against using IgM, due to lower sensitivity of IgM alone. IgA tests were not considered in the current, updated guideline because of their past poor performance and because they are rarely tested in clinical laboratories.
The current IDSA guideline includes two new recommendations. The first is a weak recommendation to use serologic assays that target nucleocapsid protein rather than spike protein when evidence of prior COVID-19 is desired. This recommendation is made to improve the specificity of testing in highly vaccinated populations, since most available vaccines result in formation of anti-spike antibodies, although some inactivated whole virus vaccines (e.g., CoronaVac and BBIBP-CorV, manufactured by Sinovac and Sinopharm, respectively) can also result in formation of anti-nucleocapsid antibodies.
The second new recommendation suggests against routine serologic testing in patients with previous SARS-CoV-2 infection or vaccination, given that no demonstrated benefits for improving patient outcomes were identified. Data were found to support higher levels of binding antibodies or the presence of neutralizing antibodies as being associated with protection against SARS-CoV-2 infection, but a single titer that predicts protection was not identified.
Summarized below are specific recommendations and comments related to the use of SARS-CoV-2 serologic testing in clinical practice. A detailed description of background, methods, evidence summary and rationales that support each recommendation can be found online and in the full text.
Recommendations
Recommendation 1: The IDSA panel recommends against using serologic testing to diagnose SARS-CoV-2 infection during the first two weeks following symptom onset (strong recommendation, low certainty of evidence).
Remarks:
- Most studies included in this recommendation’s literature review assessed unvaccinated individuals and so the panel’s findings might not apply to individuals who have been vaccinated, especially with respect to specificity, rendering test accuracy worse than presented here.
- Similarly, in today’s population there is a higher likelihood of prior SARS-CoV-2 infection than assessed in the included studies, which could also negatively affect specificity, rendering test accuracy worse than presented.
- Analogous to other respiratory viruses where non-serologic tests are preferred for diagnosis of acute infection, serologic testing should not be routinely performed for diagnosis of acute COVID-19.
Recommendation 2: The IDSA panel recommends against using IgG antibodies to provide evidence of COVID-19 in symptomatic patients with a high clinical suspicion and repeatedly negative NAAT (strong recommendation, very low certainty of evidence).
Remarks:
- Contemporary populations are more likely to have had prior SARS-CoV-2 infection or vaccination than the populations assessed in the included studies, which could negatively affect pooled specificity calculations, rendering test accuracy worse than presented here.
Recommendation 3: To assist with the diagnosis of multisystem inflammatory syndrome in children (MIS-C), the IDSA panel recommends using both IgG antibody testing and NAAT to provide evidence of current or recent past COVID-19 (strong recommendation, very low certainty of evidence).
Remarks:
- Combining IgG antibody testing and NAAT may improve both the ability to diagnose MIS-C and to rule it out, compared to using NAAT alone. Knowing the patient’s vaccination and COVID-19 history may be helpful in interpreting serology results.
- Differentiating MIS-C from conditions with overlapping symptomatology, such as Kawasaki disease, is important because Kawasaki disease requires treatment with specific therapies such as intravenous immunoglobulin or rituximab if diagnosed. The two diseases also differ in terms of potential complications and need for long-term medication and follow-up.
- The January 2023 definition of MIS-C agreed upon by U.S. Centers for Disease Control and Prevention (CDC) and other public health officials requires evidence of current or recent SARS-CoV-2 infection by PCR, antigen, or serologic testing [3].
Recommendation 4: When evidence of previous SARS-CoV-2 infection is desired, the IDSA panel suggests testing for SARS-CoV-2 IgG, IgG/IgM, or total antibodies three to five weeks after symptom onset and against testing for SARS-CoV-2 IgM (conditional recommendation, low certainty of evidence).
Remarks:
- SARS-CoV-2 NAAT and antigen tests have superior performance characteristics for diagnosis of COVID-19, compared to serologic testing.
- There are very limited situations in which SARS-CoV-2 antibody testing might be useful, e.g., in helping to diagnose MIS-C.
Recommendation 5: When evidence of prior SARS-CoV-2 infection is desired, the IDSA panel suggests using serologic assays that target nucleocapsid protein rather than spike protein (conditional recommendation, low certainty of evidence).
Remarks:
- Most available vaccines result in formation of only anti-spike antibodies but some (e.g., CoronaVac and BBIBP-CorV, manufactured by Sinovac and Sinopharm, respectively) which contain inactivated whole virus, can also result in formation of anti-nucleocapsid antibodies [4].
- Given the high prevalence of vaccination in most populations and the persistence of anti-spike antibodies over time, detection of anti-spike antibodies has low positive predictive value for diagnosis of recent COVID-19 in most individuals.
Recommendation 6: In individuals with previous SARS-CoV-2 infection or vaccination, the IDSA panel suggests against routine serologic testing given no demonstrated benefit to improving patient outcomes (conditional recommendation, very low certainty of evidence).
Remarks
- Serologic testing may be useful for diagnosing MIS-C in pediatric patients, especially when NAAT or antigen testing is negative, to provide evidence of recent COVID-19 (see Recommendation 3 above).
- A negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy such as convalescent plasma or monoclonal antibodies, if such therapies were available, or to prioritize administration of monoclonal therapies when supplies are limited.
Background
Since its emergence in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused over 760 million confirmed infections and nearly 7 million deaths worldwide [5]. Definitive diagnosis of coronavirus disease 19 (COVID-19) and asymptomatic SARS-CoV-2 infection rely on the direct detection of virus-specific RNA or virus-specific glycoprotein antigens in respiratory specimens. Serologic tests that use blood samples to detect the host antibody response to SARS-CoV-2 cannot diagnose SARS-CoV-2 infection or COVID-19, nor can they identify persons who are protected from future infection, but they may help confirm past infection. The current high seroprevalence of anti-SARS-Co-V-2 antibodies in most populations limits the utility of antibody testing for clinical or epidemiologic purposes.
Coronavirus genomes encode four major structural proteins including spike (S), envelope (E), membrane (M) and nucleocapsid (N). Both the S and N proteins of SARS-CoV-2 are immunogenic in humans; current serologic tests target antibodies directed against these antigens [6]. The S protein is the most exposed viral protein and is responsible for viral attachment and entry into host cells via binding to the angiotensin-converting enzyme 2 (ACE-2) receptor [7]. The S protein is comprised of an N-terminal S1 subunit, involved in virus-receptor binding, and a C-terminal S2 subunit involved in fusion to the host cell membrane. The S1 subunit is further divided into an N terminal domain (NTD) and a receptor binding domain (RBD). There has been particular focus on the SARS-CoV-2 RBD for vaccine development and targeted antibody therapies because neutralizing antibodies against this region effectively block viral entry [8, 9]. The N protein is an RNA-binding protein that is abundantly expressed during infection and plays a role in RNA transcription and replication [10].
There are two general types of antibodies, neutralizing antibodies (nAbs) and non-neutralizing (also known as binding antibodies) [11]. Neutralization is defined as the loss of infectivity that occurs when a nAb binds to a viral particle. Virus-specific or vaccine-induced nAbs can play a role in controlling viral infection, but definitive data is lacking to know whether individuals with detectable anti-SARS-CoV-2 nAbs are protected against reinfection. In comparison, binding antibodies are characterized by their inability to prevent viral infection of permissive cells. Regardless of their function, both types of virus-specific antibodies are potentially useful as diagnostic indicators of past infection or vaccination.
Commercially available anti-SARS-CoV-2 antibody tests use different technologies to qualitatively measure single immunoglobulin classes (IgM, IgG or IgA) or total antibody, and to differentiate nAbs from binding antibodies. IgM antibodies directed against microorganisms are typically produced first after infection and are used as a measure of recent infection. IgG antibodies generally develop later than IgM antibodies and remain elevated for months to years after infection. Although IgM antibodies can be detected within the first two weeks of symptoms in some patients, SARS-CoV-2 infection appears unusual in that IgM and IgG more commonly rise together, more than two weeks after the onset of symptoms [12]. Secretory IgA is important for mucosal immunity. IgA can also be detected systemically in certain types of infection including SARS-CoV-2, but comparatively little is known about the kinetics of IgA in blood. The components of “total antibody” presumably include IgM and IgG and theoretically other antigen-specific immunoglobulins as well.
The most common clinical diagnostic platforms utilized for SARS-CoV-2 include lateral flow (LF) devices, enzyme linked immunosorbent assays (ELISA) and chemiluminescent immunoassays (CIA). Lateral flow assays typically require a drop of blood (or serum or plasma) applied to a test strip, with results read in approximately 15-30 minutes. These devices are suitable for point-of-care testing and have potential to be deployed in the field as a part of large serological surveys. ELISA comes in a variety of different formats. Typically, a bound antigen-antibody complex is detected using a type-specific secondary antibody linked to a substrate that generates a colorimetric or fluorescent signal. CIA methods are similar to ELISA but use chemical probes that emit light instead of enzymatic substrates. Both ELISA and CIA are clinical laboratory-based methods amendable to high throughput testing using serum, plasma, or potentially dried blood spots. At this time, neutralization assays are mainly used in research settings or offered as laboratory developed tests by reference laboratories.
As of this writing, most commercially available SARS-CoV-2 antibody tests marketed in the U.S. have Emergency Use Authorization (EUA) through the U.S. Food and Drug Administration (FDA). Early in the pandemic, however, official EUA review was voluntary. Test developers were expected only to internally validate their tests and notify the FDA of their intent to market. As a result, the market was flooded with poorly performing assays. In response the FDA subsequently issued a “removed” test list that includes tests with significant performance problems, assays for which official EUA review was not appropriately submitted or assays voluntarily withdrawn by the developer. Subsequently, on September 23, 2021, the FDA revised the EUAs of certain molecular, antigen, and serology tests to establish additional Conditions of Authorization in response to the ongoing emergence of new SARS-CoV-2 variants.
The lifting of the Federal COVID-19 Public Health Emergency on May 11, 2023 was accompanied by release of a transition plan by the FDA with recommendations for marketing submission and other actions needed to enable ongoing use of COVID-19 laboratory tests that were issued EUA [13].
Many serologic tests for SARS-CoV-2 are commercially available. While most of these assays are currently available under FDA EUA, it is expected that many will soon achieve FDA clearance. The speed of development has continued to outpace rigorous assessments of test performance. In response, IDSA convened an expert panel to systematically review the available serologic literature, compare pooled estimates of test accuracy and make evidence-based recommendations for informed use in clinical practice.
Methods
This guideline was developed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for evidence assessment. This guideline serves as the second update to the original IDSA guidelines on the serologic testing of COVID-19 [14]. The list of questions addressed in the initial guideline and the current update can be found in Table s1.
Panel Composition
The panel was composed of clinicians and clinical microbiologists who are members of the Infectious Diseases Society of America (IDSA), the American Society for Microbiology (ASM), the Society for Healthcare Epidemiology of America (SHEA), and the Pediatric Infectious Diseases Society (PIDS). They represented the disciplines of infectious diseases, pediatrics, clinical microbiology, and healthcare epidemiology. The Evidence Foundation provided technical support and guideline methodologists for the development of this guideline.
Disclosure and Management of Potential Conflicts of Interest
The conflict of interest (COI) review group included two representatives from IDSA who were responsible for reviewing, evaluating and approving all disclosures. All members of the expert panel complied with the COI process for reviewing and managing conflicts of interest, which required disclosure of any financial, intellectual, or other interest that might be construed as constituting an actual, potential, or apparent conflict, regardless of relevancy to the guideline topic. The assessment of disclosed relationships for possible COI was based on the relative weight of the financial relationship (i.e., monetary amount) and the relevance of the relationship (i.e., the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The COI review group ensured that the majority of the panel and chair were without potential relevant (related to the topic) conflicts. The chair and all members of the technical team were determined to have no COIs relevant to the guidelines.
Question Generation
Clinical questions were developed into a PICO format (Population, Intervention, Comparison, Outcomes) [15] prior to the first panel meeting. Using online surveys, the panel members decided to reassess the evidence and the recommendation for 4 PICO questions that were addressed in the initial guideline in 2020 [14]. Additionally, panel members prioritized 3 new questions that have emerged as clinically important (Table s1). Because very limited data were identified to answer two of these questions, they were combined in a single recommendation (Recommendation 6.)
Search Strategy
The National Institute of Health and Care Excellence (NICE) and the Centers for Disease Control and Prevention (CDC) highly sensitive search was reviewed by the methodologists in consultation with an experienced information specialist and was determined to have high sensitivity. Terms identified in the PICO questions and the term “COVID” were added to the search strategy used for the databases PubMed, Embase, and Cochrane for antigen, molecular, and serology diagnostic test terms (Table s2). Monthly searches were performed from 2019 through January 1, 2023. During September 2022, with the help of an information specialist, improvements to the search strategy were made to reflect the covid19/sarscov2 epidemic and remove general terms like “coronavirus” which may pick up articles that are about a different virus. (Table s2). Any reference lists and literature suggested by the panelists were also reviewed throughout the guideline development process.
Screening and Study Selection
A member of the review team screened the titles and abstracts of the references identified by the search strategy and assessed if the study addressed antigen, molecular, or serologic testing. A member of the review team then assessed the studies addressing serologic testing in more detail using the full texts of the studies under consideration for potential inclusion in the review. When the inclusion of an article was debated, it was first resolved through discussion within the review team. In the case of disagreement, articles were discussed with the panel members to make the final decision.
Studies were included if they reported data on the diagnostic test accuracy of anti-SARS-CoV-2 IgM, IgG, and/or total antibody tests compared to NAAT as the reference standard. Studies were excluded if the tests evaluated were not EUA or European Commission (CE) approved, studies with fewer than 30 patients/samples, studies with incomplete test accuracy information (i.e., reported sensitivity without specificity), studies reporting atypical serology sample site collection (e.g., dried blood spots, mucosal fluids), machine learning studies, protocols, preprints, and abstracts.
Data Collection and Analysis
A member of the review team completed data extraction using a standardized data extraction form. in duplicate. Study characteristics (authors, publication year, country, study design, inclusion criteria, age, gender), index test information (timing from onset of symptoms, sample type, target antigen, platform, immunoglobulin class, FDA EUA status, and European Economic Area CE marking status), reference test (name of test, sample type), and diagnostic test accuracy results (true and false positives and negatives) were extracted.
For each study, the sensitivity and specificity of the diagnostic index test were calculated and the Clopper–Pearson method used to estimate 95% confidence intervals. We then fit the random-effects bivariate binomial model of Chu and Cole [16] to pool accuracy estimates using the glmer function of the lme4 package in R (version 4.1.2). To pool accuracy estimates for analyses including fewer than 5 studies, we fit a fixed effects model as implemented in the meta package in R (version 4.1.2). Forest plots were used to plot individual and summary estimates and conducted subgroup analyses to explore heterogeneity. To evaluate the effects of study design on results, sensitivity analyses were performed by including or excluding case control studies and assessing the effect on pooled results of test accuracy.
Risk of Bias and Certainty of Evidence
The Quality Assessment of Diagnostic Studies (QUADAS)-2 revised tool was used to assess the risk of bias in the test accuracy studies [17] (Table s3). When assessing evidence other than test accuracy in the studies of intervention, the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) was used [18] (Table s4). The GRADE framework was used to assess the overall certainty by evaluating the body of evidence for each outcome on the following domains: risk of bias, imprecision, inconsistency, indirectness, and publication bias [19, 20]. GRADE summary of findings tables were developed using the GRADEpro Guideline Development Tool [21].
Evidence to Recommendations
The panel considered the core elements of GRADE evidence in the decision process, including certainty of evidence and balance between desirable and undesirable effects. Additional domains were acknowledged where applicable (e.g., feasibility, resource use, acceptability). For all recommendations, the expert panelists reached consensus. Voting rules were agreed on prior to the panel meeting for situations when consensus could not be reached.
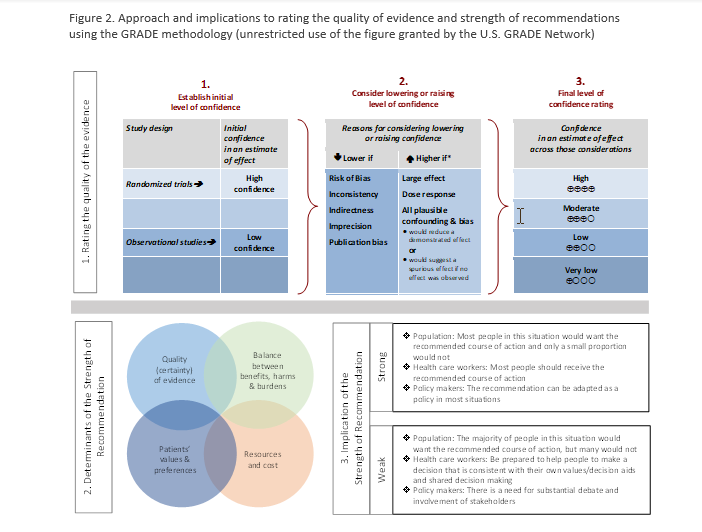
As per GRADE methodology, recommendations were labeled as “strong” or “conditional.” The words “we recommend” indicate strong recommendations and “we suggest” indicate conditional recommendations. Figure 2 provides the suggested interpretation of strong and weak recommendations for patients, clinicians, and healthcare policymakers. For recommendations where the comparators are not formally stated, the comparison of interest was implicitly referred to as “not using the test.” Some recommendations acknowledge a current “knowledge gap” and aim at avoiding premature favorable recommendations for test use and to avoid encouraging the rapid diffusion of potentially non-useful tests.
Revision Process
The draft guideline underwent review for approval by the Standards and Practice Guidelines Subcommittee of the IDSA and by the IDSA Board of Directors external to the guideline development panel. The guideline was reviewed and endorsed by ASM, PIDS and SHEA.
Updating Process
Regular, frequent screening of the literature will take place to determine the need for revisions based on the likelihood that any new data will have an impact on the recommendations. If necessary, the entire expert panel will be reconvened to discuss potential changes.
Search Results
Systematic review of the literature identified 30,645 references of which 163 informed the evidence base for this guideline recommendations (Figure s1). Characteristics of the included studies can be found in (Tables s5-s9).
Recommendation 1: Serology Testing in the First Two Weeks After Symptom Onset
Recommendation 1: The IDSA panel recommends against using serologic testing to diagnose SARS-CoV-2 infection during the first two weeks following symptom onset (strong recommendation, low certainty of evidence).
Remarks:
- Most studies included in this recommendation’s literature review assessed unvaccinated individuals and so the panel’s findings might not apply to individuals who have been vaccinated, especially with respect to specificity, rendering test accuracy worse than presented here.
- Similarly, in today’s population there is a higher likelihood of prior SARS-CoV-2 infection than assessed in the included studies, which could also negatively affect specificity, rendering test accuracy worse than presented.
- Analogous to other respiratory viruses where non-serologic tests are preferred for diagnosis of acute infection, serologic testing should not be performed routinely for diagnosis of acute COVID-19.
Summary of the evidence
We found no direct evidence of patient- or population-centered outcomes of testing versus no testing in symptomatic patients. Therefore, the panel relied on diagnostic test accuracy data to inform this recommendation.
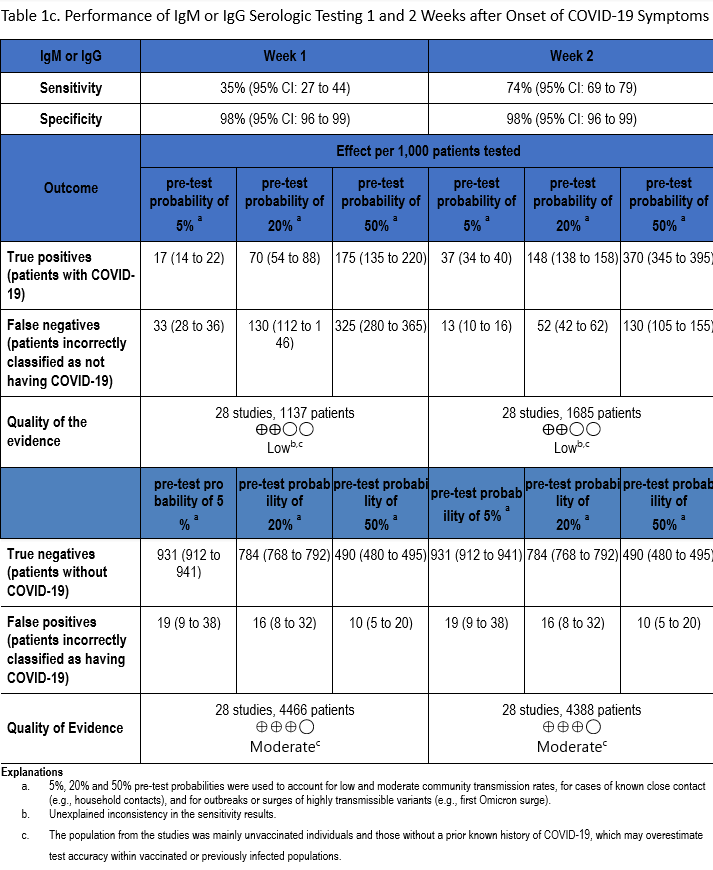
Using the applied search strategy, we identified 111 studies [22-132] that assessed diagnostic test accuracy of serologic tests (IgM, IgG, IgM or IgG) compared to a SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) test in the first two weeks after symptom onset. (Tables 1a-1c). Studies used varied sources for negative controls, including samples collected from healthy individuals, patients who had other respiratory or non-respiratory infections, patients with autoimmune diseases whose blood had been collected prior to the COVID-19 pandemic, symptomatic patients with negative NAAT for SARS-CoV-2, and/or patients hospitalized for reasons other than COVID-19. While most studies used case-control designs, some used longitudinal or cross-sectional cohort designs. For assays that detected and differentiated IgM from IgG using the same platform, an interpretation of “IgM or IgG” was considered a positive test (i.e., detection of one of the two immunoglobulins qualified as a positive test).
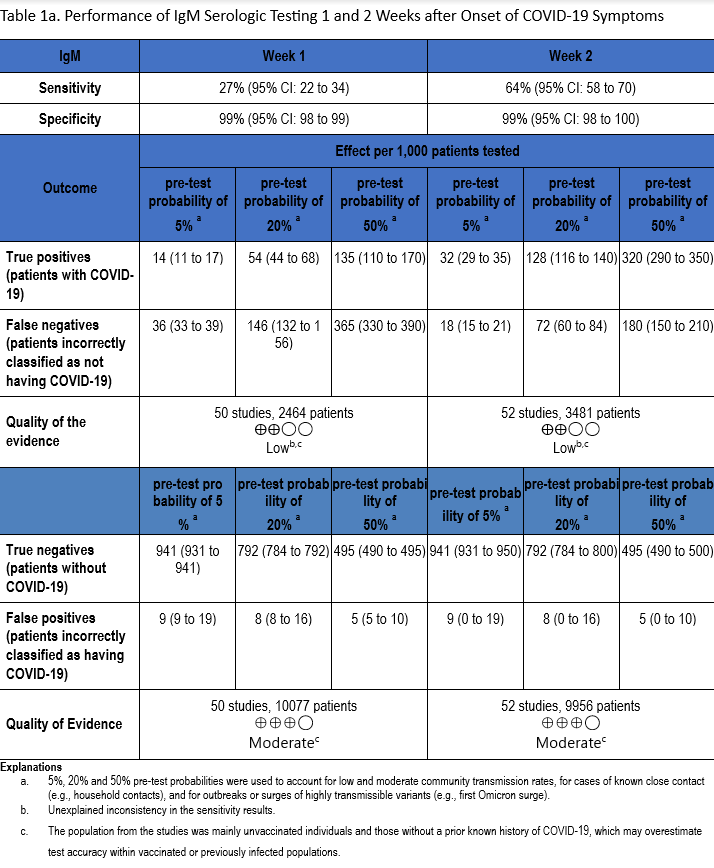
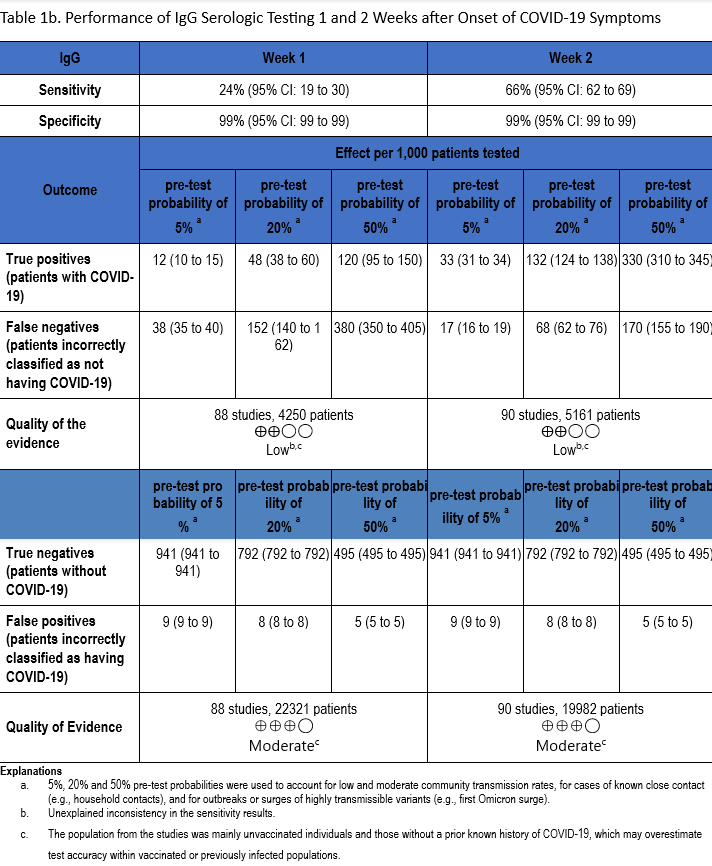
The total number of patient samples across all included studies ranged from 1,137 to 5,161 and 4,388 to 22,321, for sensitivity and specificity analyses, respectively (Figures s2a-s7b). The pooled sensitivity at one week after symptom onset ranged from 24 to 35% and at two weeks ranged from 64 to 74%, while the pooled specificity ranged from 98 to 99% (Tables 1a-1c).
The quality of evidence that informed sensitivity determinations was low, while the quality of evidence that informed specificity was moderate. Quality was rated downward for both sensitivity and specificity due to serious indirectness and unexplained inconsistency in sensitivity. Concerns with indirectness stemmed from the participants being evaluated in the pre-vaccination era, and before widespread infection. Given the very high global estimates of current SARS-CoV-2 seroprevalence, the test accuracy estimated from these studies would likely not reflect performance today.
Benefits and harms
If performed within 14 days of onset of symptomatic infection, the low sensitivity of serologic tests can lead to a high number of falsely negative results. Individuals who test falsely negative would be classified as uninfected, when, in fact, they would be infected but they would not have developed a detectable antibody response. Although the specificity of serologic testing was calculated to be 98-99%, this likely does not represent performance in vaccinated and/or previously infected individuals, who were not assessed in the included studies. Tests with low specificity can lead to false positive result for recent infection at any time point of testing. A positive result can lead to the incorrect conclusion that an individual has been recently infected, thus eliminating the search for the true etiology of their symptoms, or resulting in unnecessary treatment or isolation.
Other considerations
IgM antibody responses often occur earlier after infection compared to IgG antibody responses. In contrast, with SARS-CoV-2 infection, there does not appear to be a substantial difference in the time after infection when IgM and IgG antibodies can be detected. At one week after onset of symptoms, the pooled sensitivity for IgM tests was 27% compared to 24% for IgG tests, and at two weeks post onset of symptoms, the pooled sensitivity of IgM tests was 64% compared to 66% for IgG tests.
Conclusions and research needs for this recommendation
Serologic testing should not be performed routinely for diagnosis of acute COVID-19, analogous to the situation with other respiratory viruses. Achieving a better understanding of the role of serology as a correlate of protection with current circulating variants is a research need, especially in populations at highest risk of severe disease such as the elderly and immunocompromised. Correlation of viral RNA shedding and culture positivity (or other surrogate for infectivity) should be studied relative to immunoglobulin titers over time to help inform isolation guidelines for patients with COVID-19.
Recommendation 2: Serology Testing in Individuals with High Clinical Suspicion for SARS-CoV-2 Infection and Negative NAAT
Recommendation 2: The IDSA panel recommends against using IgG antibodies to provide evidence of COVID-19 in symptomatic patients with a high clinical suspicion and repeatedly negative NAAT (strong recommendation, very low certainty of evidence).
Remarks
- Contemporary populations are more likely to have had prior SARS-CoV-2 infection or vaccination than the populations assessed in the included studies, which could negatively affect pooled specificity calculations, rendering test accuracy worse than presented here.
Summary of the evidence
We identified 19 studies [30, 109, 130, 133-148] that compared the diagnostic test accuracy of serologic tests among patients with high clinical suspicion for COVID-19 and negative NAAT throughout the course of their illness. The percent of PCR-negative/IgG-positive test results ranged from 0% to 93% with most studies reporting this test result combination for fewer than 50% of samples. The percent of samples with PCR-negative/IgM-positive test results ranged from 2% to 83%, and the percent of samples with PCR-negative/IgM- or IgG-positive test results ranged from 11% to 62%. Finally, the percent of samples that yielded PCR-negative/total antibody-positive results ranged between 0% and 60% These studies varied in terms of assay (chemistry, method, platform) and the timing of testing (e.g., <20 days versus >15 days) (Table s6).
The overall certainty of the evidence was very low due to concerns for risk of bias (case-control study design) and imprecision (as a result of small sample size). The use of clinical symptoms as a reference standard in some studies might have resulted in misclassification (i.e., labeling non-infected patients as true positive cases based on symptoms and serology alone).
Benefits and harms
The panel’s consensus opinion was that the clinical benefit of performing SARS-CoV-2 serologic testing in patients with high clinical suspicion for COVID-19 and repeatedly negative SARS-CoV-2 NAAT is low. This is because contemporary human populations have high SARS-CoV-2 seroprevalence due to natural, vaccine-induced, or hybrid immune responses. A positive serologic result for a symptomatic patient who tests repeatedly negative by NAAT may be attributed to vaccination and/or past infection as readily as to recent SARS-CoV-2 infection. Concluding that a positive serologic test is responsible for a patient’s current symptoms can lead to misdiagnosis, particularly in periods when SARS-CoV-2 circulation is low and other respiratory virus circulation is high. In individuals who have not been vaccinated or previously infected (the population included in this recommendation’s literature review), the negative predictive value of a SARS-CoV-2 serologic test is high when performed more than 2 weeks after symptom onset. In this instance, negative serologic results should prompt further evaluation of the patients’ symptoms for alternative etiologies, if symptoms persist.
Other considerations
No SARS-CoV-2 serologic test can differentiate between recent or remote infection. Serologic tests cannot differentiate a serologic response to a vaccine antigen from a response elicited by recent or remote infection.
Conclusions and research needs for this recommendation
Assessing anti-SARS-CoV-2 antibodies provides no added benefit over repeat NAAT for the diagnosis of acute COVID-19and may lead to diagnostic uncertainty. Further research on the role of serologic and immunologic testing for diagnosis or management of long-COVID may be warranted.
Recommendation 3: Serologic Testing in Pediatric Patients with Suspicion of MIS-C
Recommendation 3: To assist with the diagnosis of multisystem inflammatory syndrome in children (MIS-C), the IDSA panel recommends using both IgG antibody testing and NAAT to provide evidence of current or recent past COVID-19 (strong recommendation, very low certainty of evidence).
Remarks:
- Combining IgG antibody testing with NAAT may improve both the ability to diagnose MIS-C and to rule it out, compared to using NAAT alone. Knowing the patient’s vaccination and COVID-19 history may be helpful in interpreting serology results.
- Differentiating MIS-C from conditions with overlapping symptomatology, such as Kawasaki disease, is important because Kawasaki disease requires treatment with specific therapies such as intravenous immunoglobulin or rituximab if diagnosed. The two diseases also differ in terms of potential complications and need for long-term medication and follow-up.
- The January 2023 definition of MIS-C agreed upon by U.S. CDC and other public health officials requires evidence of current or recent SARS-CoV-2 infection by PCR, antigen, or serologic testing [3].
Summary of the evidence
We identified 10 case series [149-158] that evaluated the use of SARS-CoV-2 serologic tests in pediatric patients presenting with signs of multisystem inflammatory syndrome in children (MIS-C) (Table s7). The case definitions of this inflammatory syndrome, typically manifesting with fever and shock similar to Kawasaki Disease, varied across studies. Most reports were from early in the pandemic and did not require laboratory evidence of SARS-CoV-2 infection to define the syndrome. However, these studies reported a higher rate of MIS-C diagnosis when IgG antibodies were added to NAAT compared to using NAAT alone. NAAT was positive in ~30-50% of cases in these studies, while IgG antibodies were positive in >80% cases in most studies. The major limitation of the evidence is that not all case series specified the timing of testing (either NAAT or serology) with respect to symptom onset. In addition, the type of serologic test utilized and epidemiologic links to COVID-19 cases were also not uniformly specified. The overall quality of evidence was very low due to the limitations of the case series study design and serious risk of bias. Even with very low certainty evidence, the panel issued a strong recommendation given the potentially catastrophic consequences of missing a diagnosis of MIS-C.
Benefits and harms
The diagnosis of MIS-C is important to provide appropriate treatment and follow up care for children, but no specific test is available to confirm this diagnosis. Another acute inflammatory syndrome in children, Kawasaki Disease, which generally affects children less than 5 years of age, can present with symptoms very similar to those of MIS-C. Although the initial treatment of both MIS-C and Kawasaki Disease typically includes supportive care and hospitalization, the differentiation of these two syndromes is important because Kawasaki disease requires hospitalization and treatment with specific therapies such as intravenous immunoglobulin or rituximab if diagnosed. The two diseases also differ in terms of potential complications and need for long term medication and follow up. The management of children with MIS-C usually requires supportive care, including hospitalization and treatment of shock and respiratory distress, evaluation of cardiac function, and administration of medications that decrease inflammation. Diagnostic tools including serology to help exclude the diagnosis of MIS-C can therefore be helpful and important, but serologic testing may not be helpful in the actual diagnosis of MIS-C as many children have serologic evidence of past COVID-19 disease and/or vaccination. Further, given the high seroprevalence of antibodies to SARS-CoV-2 in the pediatric population, a negative serologic test result provides stronger evidence against a diagnosis of MIS-C than a positive result provides evidence for MIS-C. Although the certainty of evidence to inform this recommendation was very low, the panel concluded that the potential harm associated with a misdiagnosis of MIS-C warranted a strong recommendation for serologic testing together with NAAT in suspected cases.
Other considerations
The clinical description and definition of MIS-C, as well as other SARS-CoV-2-associated inflammatory syndromes in children and adults, have varied over the pandemic and across countries and regions. Different clinical criteria with or without laboratory evidence of infection make comparisons of early studies problematic. It should be noted that the 2023 CDC definition of a confirmed case of MIS-C [3] requires meeting clinical and laboratory criteria; the latter include detection of SARS-CoV-2 RNA or antigen in a specimen up to 60 days before or during hospitalization or post-mortem, or detection of SARS-CoV-2 specific antibodies in serum, plasma, or whole blood associated with the current illness resulting in or during hospitalization [159]. Assessment of the reliability, sensitivity, and specificity of laboratory diagnostic criteria to document MIS-C will be difficult in the future because laboratory criteria are now required for diagnosis, and because case rates of MIS-C are falling. Given that pediatric seroprevalence for SARS-CoV-2 was 96.3% in December 2022, it is not surprising that 99% of recent U.S. cases of MIS-C are seropositive for SARS-CoV-2 [160].
Conclusions and research needs for this recommendation
Acute febrile inflammatory syndromes in children are challenging to diagnose, but updated definitions of MIS-C are making this diagnosis more uniform. Differentiation of Kawasaki Disease from MIS-C will continue to be an issue, particularly in young children. In addition, changes in MIS-C epidemiology have been seen, with fewer cases of MIS-C now reported in association with new SARS-CoV-2 variants [161]. In addition, COVID-19 vaccination is associated with reduced incidence of MIS-C, especially if two doses are given [162]. Continued investigation of the epidemiology and treatment of MIS-C is warranted.
Recommendation 4: Serologic Testing to Assess Previous SARS-CoV-2 Infection - Antibody Class
Recommendation 4: When evidence of previous SARS-CoV-2 infection is desired, the IDSA panel suggests testing for SARS-CoV-2 IgG, IgG/IgM, or total antibodies three to five weeks after symptom onset and suggests against testing for SARS-CoV-2 IgM (conditional recommendation, low certainty of evidence).
Remarks:
- SARS-CoV-2 NAAT and antigen tests have superior performance characteristics for diagnosis of COVID-19, compared to serologic testing.
- There are very limited situations in which SARS-CoV-2 antibody testing might be useful (e.g., in helping to diagnose MIS-C).
Summary of the evidence
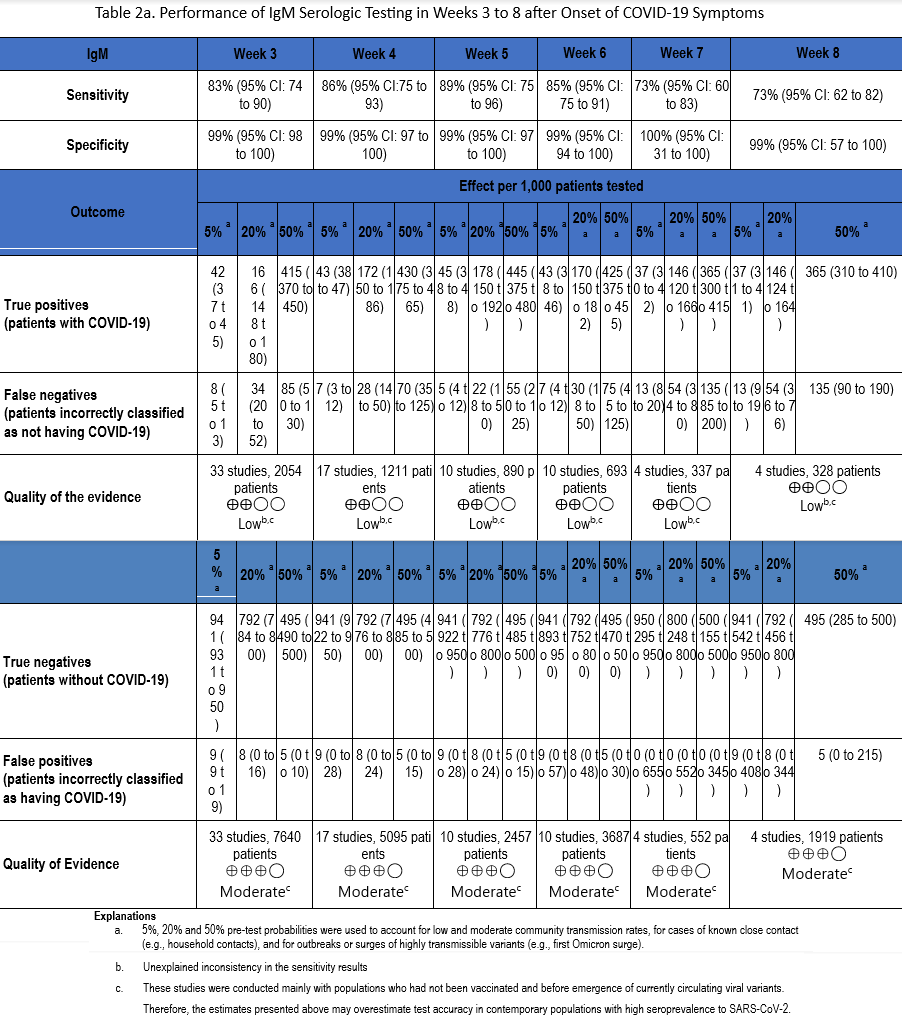
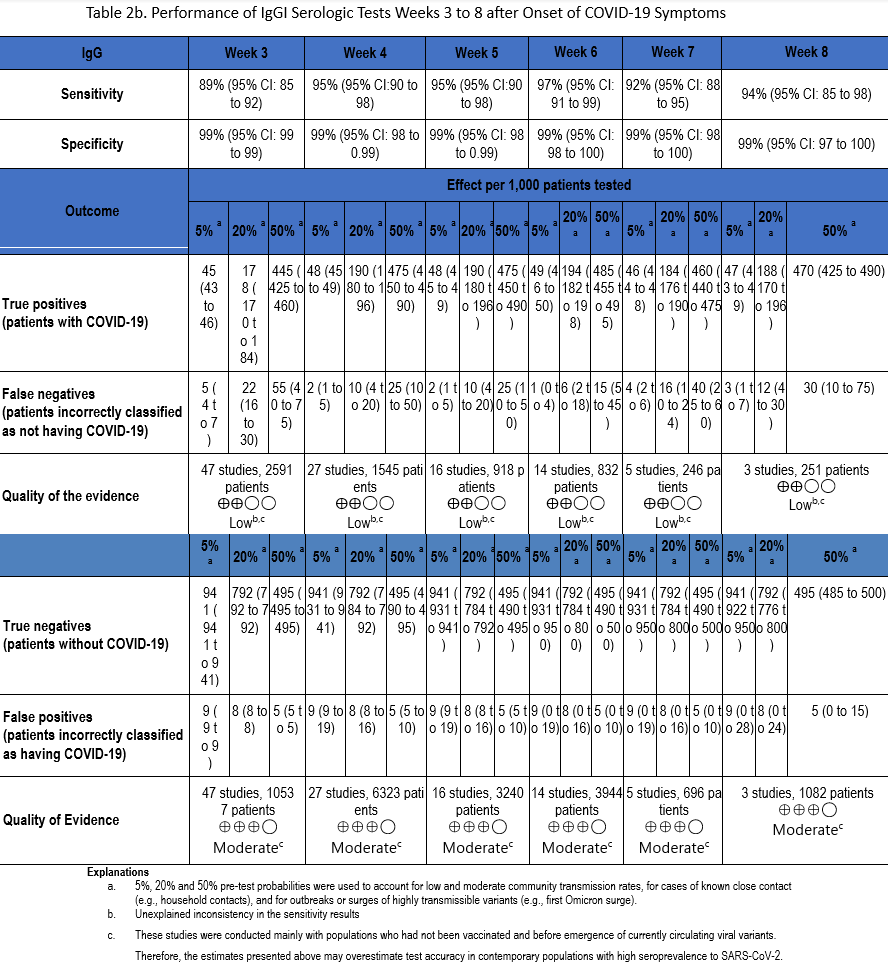
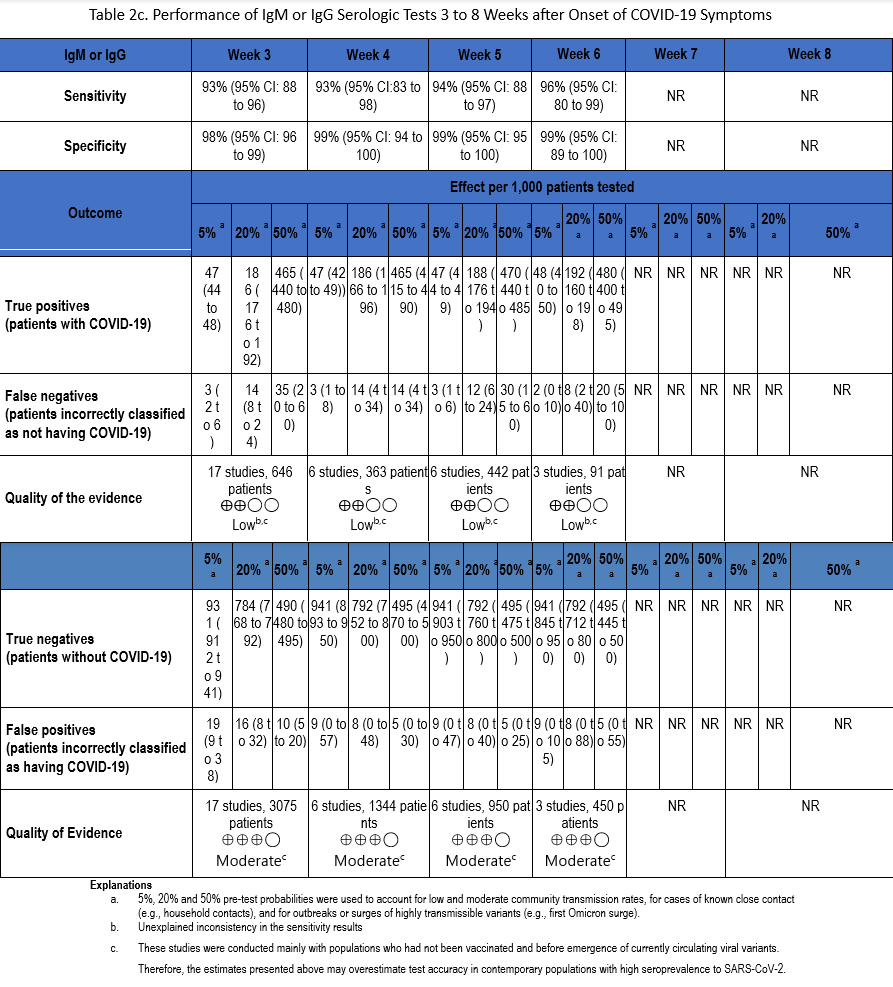
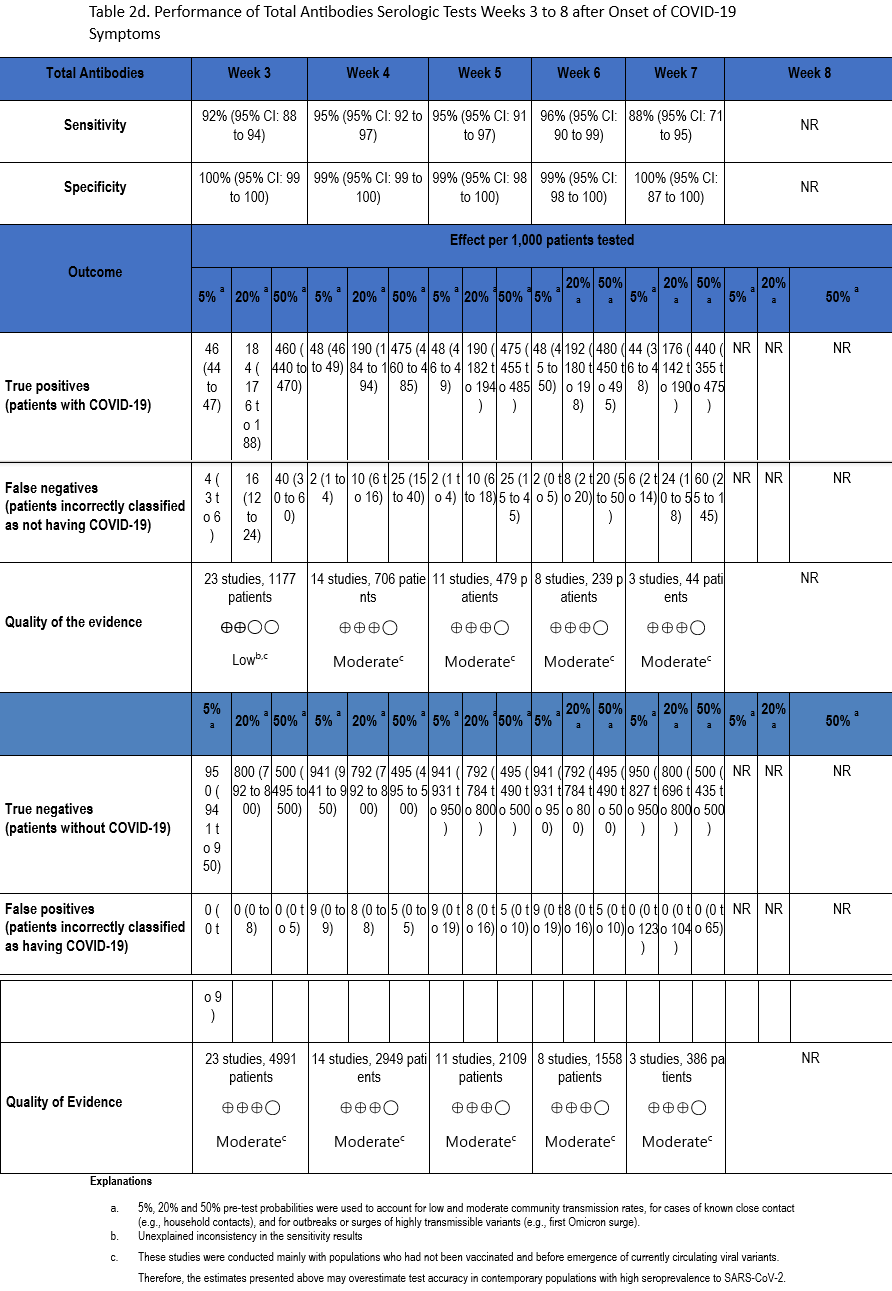
Using our search strategy, 53 studies [22-24, 29, 30, 34, 35, 38-42, 47, 51, 53, 54, 57, 58, 60-63, 66, 67, 71, 73, 76, 82, 83, 90, 91, 93, 94, 98, 102, 105, 106, 109, 110, 112, 114, 117, 118, 121, 129, 134, 163-169] were identified that assessed the diagnostic accuracy of serologic tests (IgM, IgG, IgM or IgG, and total antibodies) between weeks 3 and 8 after symptom onset compared to SARS-CoV-2 NAAT (Tables 2a-2d). Many of these same studies also informed Recommendation 1 in this guideline.
The number of samples in these studies that were included in analyses of sensitivity ranged from 44 to 2,591 and the number included in the specificity analyses ranged from 386 to 10,537 (Figures s8a-s28b). The pooled sensitivity at week three after symptom onset ranged from 83% to 93%, at week four from 86% to 95%, at week five from 89% to 95%, at week six from 85% to 97%, at week seven between 73% and 92%, and at week eight between 73% and 94%, while the pooled specificity ranged from 98% to 100% (Tables 2a-2d). When analyzed by antibody class, IgM consistently demonstrated the lowest analytical sensitivity compared with IgG alone, IgM/IgG or total antibodies.
The certainty of evidence that informed sensitivity determinations was low, while the certainty of evidence to inform specificity determinations was moderate. Quality was rated downward for both sensitivity and specificity due to serious indirectness, and due to unexplained inconsistency in sensitivity. Concerns with indirectness stemmed from the fact that study populations dated mainly from the pre-vaccination era, and preceded infection with currently circulating viral variants. Thus, the analyses presented here may overestimate test accuracy in contemporary populations with high SARS-CoV-2 seroprevalence.
Benefits and harms
There are limited data on antibody level stability after natural infection, and even less on interpretation of antibody levels in the setting of a patient who has been both vaccinated and infected. Clinicians must be aware of the limitations of antibody testing, as well as the impact of patient vaccination history. Most published studies were performed early in the pandemic, before widespread vaccination or large numbers of infected individuals. In the studies included in the literature review for this recommendation, all antibody classes had similar specificities up to 8 weeks, but the lack of sensitivity of IgM-only assays means that patients would be incorrectly classified as not having been infected.
Other considerations
Antibody testing to demonstrate prior infection may be helpful in limited scenarios. These could include evaluating patients with possible MIS-C or assessing immune response in immunocompromised patients with ongoing PCR positivity who might be candidates for monoclonal antibody or convalescent antibody therapy, if effective therapy were available, especially in setting of limited supplies.
Conclusions and research needs for this recommendation
Antibody testing for respiratory viruses is not part of routine clinical care and should not be used to guide decisions regarding vaccination or non-pharmacologic infection prevention precautions. Further study is needed to determine duration of antibody response to either vaccination or infection, and to clarify the clinical scenarios in which measuring these analytes can benefit individual patients or populations.
Recommendation 5: Serologic Testing to Assess Previous SARS-CoV-2 Infection - Antibody Target
Recommendation 5: When evidence of prior SARS-CoV-2 infection is desired, the IDSA panel suggests using serologic assays that target nucleocapsid protein rather than spike protein (conditional recommendation, low certainty of evidence).
Remarks:
- Most available vaccines result in formation of only anti-spike antibodies but some (e.g., CoronaVac and BBIBP-CorV, manufactured by Sinovac and Sinopharm, respectively) which contain inactivated whole virus, can also result in formation of anti-nucleocapsid antibodies [4].
- Given the high prevalence of vaccination in most populations and the persistence of anti-spike antibodies over time, detection of anti-spike antibodies has low positive predictive value for diagnosis of recent COVID-19 in most individuals.
Summary of the evidence
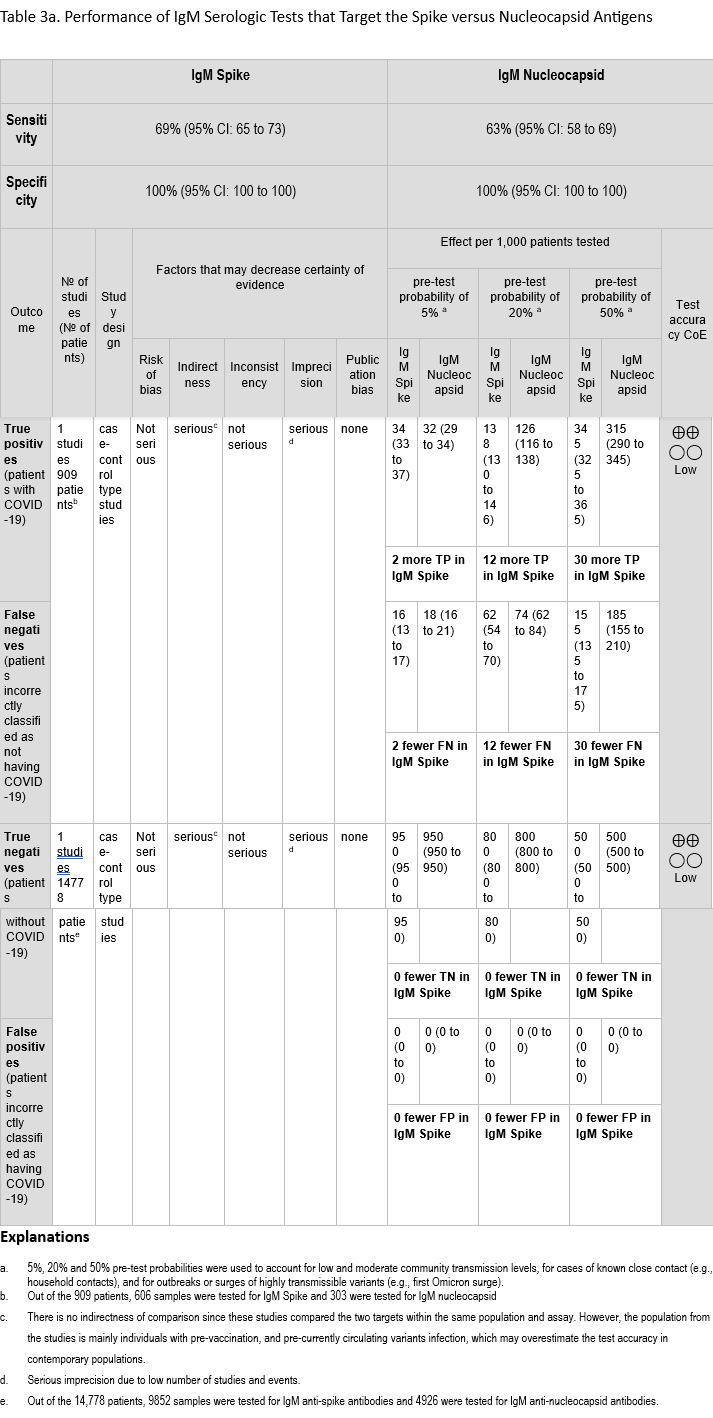
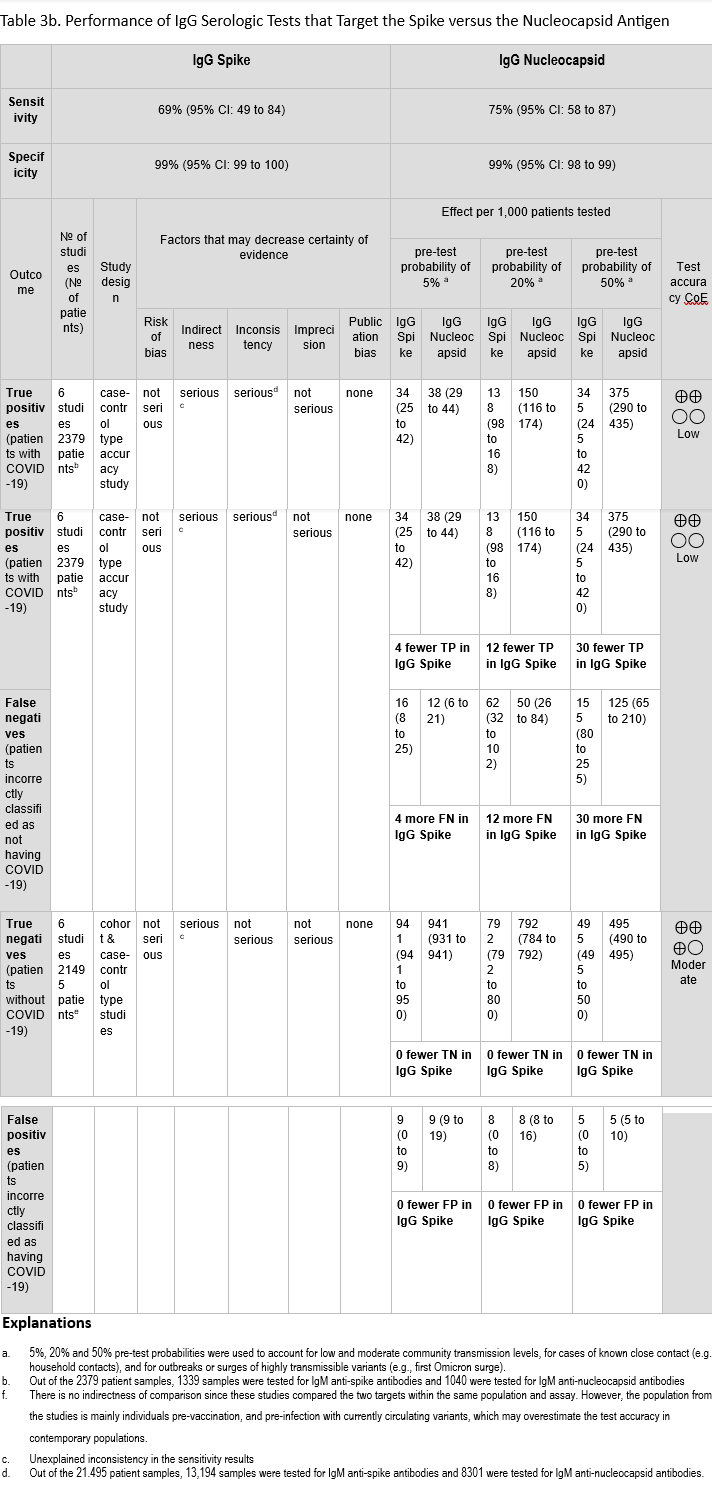
We identified 6 studies [55, 69, 109, 170-172] that assessed in the same study population the diagnostic test accuracy of serologic tests that target spike and nucleocapsid proteins and compared the results of a recent (past 1-8 weeks) positive SARS-CoV-2 NAAT (Tables 3a-3b). All six studies included results for IgG, and one study also assessed IgM [171]. The sensitivity of assays targeting spike were 69% (95% CI: 65 to 73) and 69% (95% CI: 49 to 84) for IgM and IgG, respectively. The sensitivity of assays targeting nucleocapsid were 63% (95% CI: 58 to 69) and 75% (95% CI: 58 to 87) for IgM and IgG, respectively. The specificity ranged between 99% and 100% for both targets with IgM and IgG (Figures 29a-30b).
The certainty of evidence that informed sensitivity and specificity for IgM was low, and the certainty of evidence that informed sensitivity and specificity for IgG was low and moderate, respectively. The certainty was downgraded for concerns related to indirectness and imprecision (single study for IgM) and unexplained inconsistency (sensitivity of IgG). Indirectness is a concern because these studies were conducted primarily in an unvaccinated population and with SARS-CoV-2 variants that no longer predominate in the population. Together, these factors may overestimate test accuracy for the current population, which has high seroprevalance for SARS-CoV-2 due to vaccination and/or natural infection. We did not lower the certainty for indirectness of comparison as the studies tested the same population with the same anti-spike and anti-nucleocapsid antibody assays.
Benefits and harms
Regardless of target, serologic assays are likely to underestimate recent infection by SARS-CoV-2. While specificity was high in the studies evaluated, studies were conducted before widespread SARS-CoV-2 vaccination and infection. Specificity of anti-spike IgG as a marker of prior infection is expected to be low when testing the current, highly vaccinated population, as the spike antigen is the target for most available vaccines. In contrast, a positive result for IgM or IgG nucleocapsid antibodies is indicative of prior infection in most individuals, with the exception of those who received a vaccine that resulted in formation of anti-nucleocapsid antibodies, such as the inactivated whole-virus vaccines available outside the U.S.
Other considerations
While not specifically evaluated, waning of antibody levels over time after natural infection and vaccination has been noted. In particular, nucleocapsid-specific antibodies may be more short-lived in both immunocompetent [173]and immunocompromised patients [174], although comparing results between studies is often difficult due to the different antibody assays used. As such, timing of antibody testing is critical to the negative predictive value of the test and should ideally be performed within 3-4 weeks of symptom onset.
Conclusions and research needs for this recommendation
Sensitivity and specificity of nucleocapsid and spike antibody tests are similar, although interpretation of results as indicative of past infection (anti-nucleocapsid) versus past infection or vaccination (anti-spike) requires that clinicians know and understand the antibody target of the test that is used. While NAAT remains the recommended approach for diagnosis of COVID-19, detection of anti-nucleocapsid IgG, combination IgG/IgM, or total antibodies may be useful for determining recent past infection in selected clinical situations, e.g., MIS-C (see Recommendation 3 above.)
Recommendation 6: Serologic Testing to Improve Outcomes in Individuals with History of Vaccination or Previous Infection
Recommendation 6: In individuals with previous SARS-CoV-2 infection or vaccination, the IDSA panel suggests against routine serologic testing given no demonstrated benefit to improving patient outcomes (conditional recommendation, very low certainty of evidence).
Remarks
- Serologic testing may be useful for diagnosing MIS-C in pediatric patients, especially when NAAT or antigen testing is negative, to provide evidence of recent COVID-19 (see Recommendation 3 above).
- A negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy such as convalescent plasma or monoclonal antibodies, if such therapies were available, or to prioritize administration of monoclonal therapies when supplies are limited.
Summary of Evidence
Studies addressing natural infection:
We found no direct evidence to correlate serologic testing with improved outcomes at the population or individual level for persons with prior COVID-19. We identified 6 studies [175-180], 5 cohort and 1 case control study [177] that evaluated the relationship between antibody level (e.g., seropositivity) and decreased SARS-CoV-2 reinfection rates. This included five studies that assessed binding antibodies and one that assessed neutralizing antibodies [175]. Across the cohort studies, subjects with a seropositive result post-infection appeared to have a lower likelihood of developing re-infection. In the case control study [177], doubling of the antibody level was associated with reduced odds of reinfection. These studies, however, varied in the definition of reinfection, the timing of testing in relation to history of COVID-19, the antibody test used, and the comparison groups. (Table s8).
Studies addressing vaccination:
We identified 10 post-vaccination studies [181-190] including 6 cohort studies, 1 clinical trial [184], 1 systematic review [183], and 2 case control studies [182, 189] that evaluated the relationship between binding antibody (bAb) level and SARS-CoV-2 breakthrough infection rates [189, 190]. Results were inconsistent: 5 studies reported a correlation between seropositivity and decreased SARS-CoV-2 infection rates, while 2 [187, 188] reported no difference in infection rates between those who had seroconverted after vaccination and those who had not. Of note, the number of breakthrough infections in some studies was small, and several studies focused on patient populations with immunocompromising conditions.
The presence of neutralizing antibodies (nAbs) appears to correlate with protection against breakthrough infection based on a clinical trial and a systematic review (Table s9). However, a single tier or threshold antibody level that is predictive of protection has not been identified, and unless calibrated to a common reference standard, quantitative results are not commutable across assays (See Other Considerations below). We also did not identify any studies measuring the predictive value of hybrid immunity (i.e., serologic testing in individuals with confirmed previous SARS-CoV-2 infection and COVID-19 vaccination).
Summary statement:
The overall quality of evidence was very low due to risk of bias (Table s4), indirectness, and imprecision. There is indirectness in comparison, as the included studies only describe differences between subjects who underwent serologic testing. It is unclear if patient outcomes are any different because of testing (i.e., if test results would lead to any meaningful changes in care or outcomes). It is unclear therefore if serologic testing provides any benefit for patient care decisions or to patients.
Benefits and Harms
Anti-SARS C0V-2 antibody tests help identify people who may have been infected with SARS-CoV-2 or were vaccinated. However, a universal antibody level that is predictive of protection from re-infection after infection or vaccination has not been identified. The panel did not identify any benefits to antibody testing in most individuals to inform management decisions.
There may be selected scenarios for immunocompromised hosts, however, where serologic testing could be considered. One example is for patients with symptomatic COVID-19 and significant humoral immune impairment who are being evaluated for treatment with convalescent plasma. The European Conference of Infections in Leukemia (ECIL-9) as well as the National Comprehensive Cancer Network (NCCN) recommend consideration of convalescent plasma for hematologic/BMT patients who are SARS-CoV-2 seronegative [191] [192-194]. An additional use of serology is for prioritization of new monoclonal antibody therapies, if they become available in the future, especially if supplies are limited. Prioritizing treatment for those individuals most in need of protection could help optimize utilization of resources. Of note, a thorough review of convalescent plasma or monoclonal antibody therapies for COVID-19 is outside of the scope of this guideline.
Positive anti-SARS-CoV-2 antibody testing is part of the CDC case definition for MIS-C [195]. However, false positive results may detract from identifying the true cause of illness. For example, for children it is important to differentiate MIS-C from other inflammatory processes such as bacterial infections, Kawasaki Disease or rheumatic fever because the management and long term follow up of these entities is different. There are also potential harms to serologic testing after vaccination. False positive results may promote a false sense of security and lead individuals to take fewer steps to protect themselves against SARS-CoV-2 or to not seek treatment when it would otherwise have been indicated.
False negative results are also possible, which could cause emotional distress for previously vaccinated individuals by leading them to assume that the vaccine was ineffective. Post-vaccination antibody test results may be negative in individuals without a history of previous natural infection if the test used does not detect the type of antibodies induced by the vaccine.
Other considerations
While current EUA indications do not preclude the use of SARS-CoV2 antibody tests in vaccinated individuals, none of the currently authorized tests has been authorized to assess humoral immunity post-vaccination or to define a threshold of protection after infection. Therefore, the FDA recommends against SARS-CoV-2 antibody testing to assess immunity.
Detection of anti-RBD-bAbs has generally correlated with the emergence of nAbs and the detection of nAbs has been associated with protection against COVID-19. Vaccine clinical trial data also showed that higher antibody levels (either bAbs or nAbs) were associated with fewer breakthrough infections. Despite these observations, a definitive correlate of protection has not been identified. One complicating factor is that antibody levels measured across different studies used different antibody tests that had not been calibrated to a common reference standard such as the WHO international standard for anti-SARS-CoV-2 immunoglobulin [196]. Currently, only one commercial test has been authorized to detect nAbs from individuals with recent or prior SARS-CoV-2 infection [197]. While this assay has been calibrated against the WHO standard, it was not validated for testing post-vaccination or for predicting protection from re-infection. In addition, a given antibody titer may provide lower protection against variants compared with the strains included in the vaccine or that caused prior infection. Furthermore, serologic testing does not assess the full spectrum of immune responses to infection or vaccination. Induction of T-cell immunity plays a critical role in protection against SARS-CoV-2, and while the potential importance of viral mutations and T-cell immune evasion is debated, it is unlikely that variants will be able to evade SARS-CoV-2-specific cell mediated immunity. Currently, assays designed to quantify T-cell immune function are not widely available for clinical use.
Conclusions and research needs for this recommendation
Higher antibody levels generally correlate with protection against SARS-CoV-2 infection, but a single titer that predicts protection has not yet been identified. Therefore, antibody testing (either with bAns or nAbs) to assess for immunity to SARS-CoV-2 is not recommended routinely. Antibody testing should not be used to guide vaccination, re-vaccination or booster decisions.
More research is needed to determine the role of SARS-CoV-2 antibody testing in evaluating immunity against SARS-CoV-2 and to better understand if antibody tests will be helpful for identifying “non-responders” who should receive repeated COVID-19 vaccination or a different schedule for boosters. Efforts to better predict antibody longevity, correlation of bAb levels to nAbs levels, and serological surrogates of immune protection (including how this may differ in individuals who also have a history of both natural COVID-19 and vaccination) are needed. Future studies should use standardized assays that are- calibrated to an international standard.
Discussion
Early in the COVID-19 pandemic, there was intense interest in measuring antibody responses to SARS-CoV-2 infection, with the hope that a correlate of protective immunity would be identified. Similar interest accompanied release of results of clinical trials of the first SARS-CoV-2 vaccines. In response, intensive and wide-ranging research into the immunology of SARS-CoV-2 was conducted. This work yielded important insights, including that prior infection and vaccination reduce the risk of severe and fatal COVID-19, and that the presence of neutralizing antibodies (versus their absence) and higher binding antibody concentrations (versus lower concentrations) provide greater risk reduction. Yet despite these discoveries, and after almost 800 million infections and administration of 13.5 billion vaccine doses [5], the hope that a serologic marker of protection will be found remains unrealized. Consequently, current clinical indications for testing SARS-CoV-2 antibodies are few. In addition, as seroprevalence approaches 100% in most human populations, measuring antibodies has declined in importance as a public health strategy to surveille for SARS-CoV-2 infection.
In the current guideline, the IDSA panel identified only one clinical situation in which antibody testing is recommended: to assist with the diagnosis of MIS-C in children. Of note, ongoing assessment of the value of serologic testing in this clinical setting will be difficult since a positive test result is included in the case definition [3]. The panel also acknowledges that a negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy such as convalescent plasma, monoclonal antibodies, or to prioritize administration of monoclonal therapies when supplies are limited.
To optimize diagnostic test accuracy when considering serologic testing, IgG, IgG/IgM, or total antibody assays are suggested over an IgM assay, due to reduced sensitivity of IgM alone. Testing should not be done during the first two weeks of symptoms, since antibody production is often not detectable until week 3 or later; of note, few data are available after 8 weeks of symptoms, and the panel did not assess studies that reported results after this time. If serology is being done to assess for evidence of previous SARS-CoV-2 infection in someone who has been vaccinated with an mRNA (e.g., Moderna COVID-19 vaccine), protein subunit (e.g. Novavax COVID-19 vaccine), or viral vector vaccine (e.g., J&J/Janssen COVID-19 vaccine, which is no longer available for use in the U.S.), serologic assays that target the nucleocapsid protein are preferred over those that target the spike protein, since these vaccines elicit anti-spike antibodies.
It is important to emphasize that serologic test performance remains variable and sometimes the same manufacturer’s assay performed quite differently across studies. Factors that may have contributed to heterogeneity across studies include differences in the patient population tested, the exact timing of testing relative to symptom onset and the use of different NAAT assays as the reference standard for comparison. Patients with mild symptoms, or those who are asymptomatic [198], may display weaker immune responses to SARS-CoV-2 compared to those with severe illness. Standardized reporting of disease severity was not included in most studies. The exact timing of testing was also not specified in all studies. Uncertainly around the timing of testing could have affected the time-stratified analyses. In addition, most studies did not include an international standard. Future assays should be calibrated against an international reference standard such as that developed by the WHO [199]. While in the previous version of this guideline, the clinical performance of LF assays was more variable than ELISA or CIA tests, this difference was not observed in the current updated literature review. Finally, in a new subgroup analysis, we found no substantive differences in test characteristics (i.e., sensitivity and specificity) based on viral antigen (i.e, spike vs nucleocapsid protein).
Conclusions
Clinicians and public health officials need to understand the indications for serologic testing and the performance of serology assays used in their settings to accurately interpret anti-SARS-CoV-2 antibody test results. Whenever possible, serologic assays with established high sensitivity and specificity (i.e., ≥99.5%) should be employed. IgG, IgG/IgM and total antibody tests appear to have better sensitivity and specificity than other immunoglobulin classes and perform best when used between three to five weeks after symptom onset. The clinical indications for antibody testing to support a diagnosis of COVID-19 are limited. Antibodies to SARS-CoV-2 support the diagnosis of MIS-C in children. A negative spike antibody test may be a useful metric to identify immunocompromised patients who are candidates for immune therapy such as convalescent plasma, monoclonal antibodies, or to prioritize administration of monoclonal therapies when supplies are limited. The current high seroprevalence of anti-SARS-CoV-2 antibodies in most populations reduces the value of antibody testing for epidemiologic purposes. While higher antibody titers and presence of neutralizing antibodies correlate with reduced risk of SARS-CoV-2 infection, a correlate of immune protection from infection has not been defined.
Notes
Financial Support
This project was funded in part by a cooperative agreement with the Centers for Disease Control and Prevention (grant number 6 NU50CK000477-04-01). The Centers for Disease Control and Prevention is an agency within the Department of Health and Human Services (HHS). The contents of this guideline do not necessarily represent the policy of CDC or HHS and should not be considered an endorsement by the Federal Government.
COI Summary
The following list displays what has been reported to the IDSA. To provide thorough transparency, the IDSA requires full disclosure of all relationships, regardless of relevancy to the guideline topic. Evaluation of such relationships as potential conflicts of interest is determined by a review process which includes assessment by the Board of Directors liaison to the Standards and Practice Guideline Committee and, if necessary, the Conflicts of Interest (COI) and Ethics Committee. The assessment of disclosed relationships for possible COI is based on the relative weight of the financial relationship (i.e., monetary amount) and the relevance of the relationship (i.e., the degree to which an association might reasonably be interpreted by an independent observer as related to the topic or recommendation of consideration). The reader of these guidelines should be mindful of this when the list of disclosures is reviewed. M.H. serves on a clinical adjudication panel for Sanofi; receives research funding from the Centers for Disease Control and Prevention (CDC) and CDC Foundation; serves on the Society for Healthcare Epidemiology of America (SHEA) Board of Directors and Chair of the SHEA Education & Research Foundation; received other numerations from Sage, Medline, and Molnylycke; and served as Chair of the IDSA Diagnostics Committee. K.H. served an advisor to Quidel, BioFire, Pfizer, and Takeda; received other numerations from Quidel, Pfizer and Takeda; served Editor to American Society of Microbiology (ASM) and member of Clinical and Laboratory Standards Institute Antifungal Committee; received research funding from the National Institutes of Health (NIH); and served on the exam committee for the American Board of Internal Medicine, and associate editor for Open Forum Infectious Diseases. J.E. serves as a consultant for Sanofi Pasteur, Pfizer, and AstraZeneca; an advisor/consultant for Meissa Vaccines; receives research funding from the CDC, Pfizer, Brotman Baty Research Institute, Merck, Novavax, GlaxoSmithKline, and AstraZeneca; served as an advisor to Teva Pharmaceuticals; and served as member of Pediatric Infectious Diseases Society (PIDS) Publication Committee and Transplant ID Committee. R.H. serves on the advisory board for Specific Diagnostics; serves as an advisor for ThermoFisher, Qiagen, Torus, and Roche; serves as an editor for the Journal of Clinical Microbiology (JCM); serves as a member of the microbiology committee for the College of American Pathology and a member of the AST subcommittee for the Clinical and Laboratory Standards Institute (CLSI); serves as Vice-chair for the Diagnostics Committee at the Infectious Diseases Society of America (IDSA); serves on the Professional Practices Group at the American Society for Microbiology (ASM); receives research funding from Qiagen, bioMerieux, Momentum Diagnostics, Specific Diagnostics, IHMA and Pattern; served as a consultant for bioMerieux. M.L. serves as an advisor for Sanofi, Seqirus, Medicago, GSK, Janssen, Novavax, Pfizer, MD Brief; receives research funding from the Canadian Institutes of Health Research, World Health Organization (WHO), Medical Research Council (United Kingdom), has received in-kind supply of vaccine from Sanofi, has been paid for expert testimony on institutional and workplace vaccine policy, and has served on the DSMB for CanSino Biologics and an advisor to Merck. D.M. serves as a member of the direct taskforce for diagnostic stewardship for the Society for Healthcare Epidemiology of America (SHEA); receives research funding from the Centers for Disease Control and Prevention (CDC), National Institute of Health (NIH), Veterans Affairs, Health Services Research and Development (HRSD) and the Agency for Healthcare Research and Quality (AHRQ). R.P. has a patent on Bordetella pertussis/parapertussis PCR issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, and a patent on an anti-biofilm substance issued; serves as consultant to PhAST, Torus Biosystems, Day Zero Diagnostics, Mammoth Biosciences, Netflix, Abbott Laboratories, Oxford Nanopore Technologies, CARB-X, Qvella, and HealthTrackRx; receives other numeration from NBME, UpToDate, and the Infectious Disease Board Review Course; received grants from CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate, ContraFect, TenNor Therapeutics Limited, Shionogi, NIH, BIOFIRE, Adaptive Phage Therapeutics, National Science Foundation, and the Department of Defense; and has served as a consultant to Curetis, Specific Technologies, NextGen Diagnostics, Pathoquest, Selux Diagnositcs, and 1928 Diagnostics. S.S. serves as a Board member for the Evidence Foundation, receives honoraria for evidence reviews, methodological support and teaching from the Evidence Foundation; serves on guideline panels for the American Gastroenterological Association (AGA); and receives research funding from the Department of Veterans Affairs Evidence Synthesis Program. Y.F.Y. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support and teaching from the Evidence Foundation, the AGA for evidence reviews, and the Institute for Clinical and Economic Review (ICER) for committee meetings; serves as a Director for the Evidence Foundation and for the U.S. GRADE Network; and served on an Independent Appraisal Committee for ICER. R.M. serves as a Board member for the Evidence Foundation; and receives honoraria for evidence reviews, methodological support and teaching from the Evidence Foundation. M.H.M. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support and teaching from the Evidence Foundation; receives research funding from the Agency for Healthcare Research and Quality, the Endocrine Society, and the Society for Vascular Surgery; has received research funding the American Society of Hematology and the WHO; and has served as a guideline methodologist for the WHO. A.B. received honorarium from the ICER. R.A.M. serves as a Board member for the Evidence Foundation; receives honoraria for evidence reviews, methodological support and teaching from the Evidence Foundation, and ICER for committee meetings; receives research funding from the NIH, the WHO, the American College of Rheumatology, the American Society of Hematology, and Bohringer Ingelheim; serves as Chair of the Midwest Comparative Effectiveness Public Advisory Council of the ICER; serves on the Methods Committee for Kidney Disease Improving Global Outcomes Work Group; serves on the Clinical Guidelines Committee for the Canadian Society of Nephrology; and previously served on the Clinical Guidelines Committee for the American College of Physicians (ACP). All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed. All other authors report no potential conflicts.
IDSA Disclaimer
It is important to realize that guidelines cannot account for individual variation among patients. They are not intended to supplant physician judgment with respect to particular patients or special clinical situations. IDSA considers adherence to these guidelines to be voluntary, with the ultimate determination regarding their application to be made by the physician in the light of a patient’s individual circumstances. While IDSA makes every effort to present accurate and reliable information, the information provided in these guidelines is “as is” without any warranty of accuracy, reliability, or otherwise, either express or implied. Neither IDSA nor its officers, directors, members, employees, or agents will be liable for any loss, damage, or claim with respect to any liabilities, including direct, special, indirect, or consequential damages, incurred in connection with implementation of these guidelines or reliance on the information presented.
The guidelines represent the proprietary and copyrighted property of IDSA. Copyright 2024 Infectious Diseases Society of America. All rights reserved. No part of these guidelines may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of IDSA. Permission is granted to physicians and health care providers solely to copy and use the guidelines in their professional practices and clinical decision-making. No license or permission is granted to any person or entity, and prior written authorization by IDSA is required, to sell, distribute, or modify the guidelines, or to make derivative works of or incorporate the guidelines into any product, including but not limited to clinical decision support software or any other software product. Except for the permission granted above, any person or entity desiring to use the guidelines in any way must contact IDSA for approval in accordance with the terms and conditions of third-party use, in particular any use of the guidelines in any software product.
References
- Centers for Disease Control and Prevention. Nationwide COVID-19 Infection and Vaccination Induced Antibody Seroprevalence (Blood donations). Available at: https://covid.cdc.gov/covid-data-tracker/#nationwide-blood-donor-seroprevalence-2022 Accessed 01 August 2023
- Bergeri I, Whelan MG, Ware H, et al. Global SARS-CoV-2 seroprevalence from January 2020 to April 2022: A systematic review and meta-analysis of standardized population-based studies. PLoS Med 2022; 19(11): e1004107.
- Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Available at: https://www.cdc.gov/mis/mis-c/hcp/index.html. Accessed 1 January 2023 and 20 August 2023
- Dinc HO, Saltoglu N, Can G, et al. Inactive SARS-CoV-2 vaccine generates high antibody responses in healthcare workers with and without prior infection. Vaccine 2022; 40(1): 52-8.
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/#:~:text=Globally%2C%20as%20of%201%3A56pm,vaccine%20doses%20have%20been%20administered. Accessed 03 August 2023 and 27 August 2023
- Premkumar L, Segovia-Chumbez B, Jadi R, et al. The receptor-binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Science Immunology 2020; 5(48): eabc8413.
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020; 181(2): 281-92.e6.
- Poh CM, Carissimo G, Wang B, et al. Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nature Communications 2020; 11(1): 2806.
- Jiang S, Hillyer C, Du L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol 2020; 41(5): 355-9.
- Dutta NK, Mazumdar K, Gordy JT. The Nucleocapsid Protein of SARS–CoV-2: a Target for Vaccine Development. Journal of Virology 2020; 94(13): e00647-20.
- Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nature Reviews Immunology 2006; 6(3): 231-43.
- Lee CY-P, Lin RTP, Renia L, Ng LFP. Serological Approaches for COVID-19: Epidemiologic Perspective on Surveillance and Control. Front Immunol 2020; 11: 879-.
- Administration USFD. Transition Plan for Medical Devices Issued Emergency Use Authorizations (EUAs) Related to Coronavirus Disease 2019 (COVID-19). Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/transition-plan-medical-devices-issued-emergency-use-authorizations-euas-related-coronavirus-disease. Accessed 20 August 2023
- Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America Guidelines on the Diagnosis of COVID-19:Serologic Testing. Clin Infect Dis 2020.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64(4): 395-400.
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. J Clin Epidemiol 2006; 59(12): 1331-2; author reply 2-3.
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155(8): 529-36.
- Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919.
- Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 21 part 1. Study design, risk of bias, and indirectness in rating the certainty across a body of evidence for test accuracy. J Clin Epidemiol 2020; 122: 129-41.
- Schünemann HJ, Mustafa RA, Brozek J, et al. GRADE guidelines: 21 part 2. Test accuracy: inconsistency, imprecision, publication bias, and other domains for rating the certainty of evidence and presenting it in evidence profiles and summary of findings tables. J Clin Epidemiol 2020; 122: 142-52.
- GRADEpro GDT. GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc.). Available at: https://gradepro.org/.
- Narasimhan M, Mahimainathan L, Araj E, et al. Clinical evaluation of the abbott alinity sars-cov-2 spike-specific quantitative igg and igm assays among infected, recovered, and vaccinated groups. Journal of Clinical Microbiology 2021; 59(7).
- Hibino M, Watanabe S, Tobe S, et al. Antibody responses to SARS-CoV-2 nucleocapsid and spike proteins in hospitalized patients with COVID-19: A multicenter, retrospective, cross-sectional study in Japan. Respir Investig 2022; 60(2): 256-63.
- Maine GNL, K. M.: Krishnan, S. M.: Afolayan-Oloye, O.: Fatemi, S.: Kumar, S.: VanHorn, L.: Hurand, A.: Sykes, E.: Sun, Q. Longitudinal characterization of the IgM and IgG humoral response in symptomatic COVID-19 patients using the Abbott Architect. J Clin Virol 2020; 133: 104663.
- Cs L, Sp H, Yl L, Sk P, Tc A. Performance of an automated chemiluminescent immunoassay for SARS-COV-2 IgM and head-to-head comparison of Abbott and Roche COVID-19 antibody assays. Practical Laboratory Medicine 2021; 25.
- Interiano CM, S.: Turner, B.: Gonzalez, M.: Rogers, B.: Jerris, R.: Weinzierl, E.: Elkhalifa, M.: Leung-Pineda, V. Longitudinal evaluation of the Abbott ARCHITECT SARS-CoV-2 IgM and IgG assays in a pediatric population. Practical Laboratory Medicine 2021; 25.
- Higgins VF, A.: Wang, X. Y.: Bhandari, M.: Daghfal, D. J.: Kulasingam, V. Anti-SARS-CoV-2 IgM improves clinical sensitivity early in disease course. Clin Biochem 2021; 90: 1-7.
- Conklin SEM, K.: Manabe, Y. C.: Schmidt, H. A.: Miller, J.: Keruly, M.: Klock, E.: Kirby, C. S.: Baker, O. R.: Fernandez, R. E.: Eby, Y. J.: Hardick, J.: Shaw-Saliba, K.: Rothman, R. E.: Caturegli, P. P.: Redd, A. D.: Tobian, A. A. R.: Bloch, E. M.: Larman, H. B.: Quinn, T. C.: Clarke, W.: Laeyendecker, O. Evaluation of Serological SARS-CoV-2 Lateral Flow Assays for Rapid Point-of-Care Testing. J Clin Microbiol 2021; 59(2).
- Fujigaki HT, M.: Osawa, M.: Sakurai, A.: Nakamoto, K.: Seto, K.: Fujita, T.: Hata, T.: Akiyama, H.: Doi, Y.: Saito, K. Reliability of serological tests for COVID-19: comparison of three immunochromatography test kits for SARS-CoV-2 antibodies. Heliyon 2020; 6(9): e04929.
- Pérez-García FP-T, R.: Romanyk, J.: Arroyo, T.: Gómez-Herruz, P.: Cuadros-González, J. Alltest rapid lateral flow immunoassays is reliable in diagnosing SARS-CoV-2 infection from 14 days after symptom onset: A prospective single-center study. J Clin Virol 2020; 129: 104473.
- Serrano MMR, D. N.: Palop, N. T.: Arenas, R. O.: Córdoba, M. M.: Mochón, M. D. O.: Cardona, C. G. Comparison of commercial lateral flow immunoassays and ELISA for SARS-CoV-2 antibody detection. J Clin Virol 2020; 129: 104529.
- Jung JG, E.: Jariwala, P.: Pham, H.: Huang, R.: Benzi, E.: Issaq, N.: Matzuk, M.: Singh, I.: Devaraj, S. Clinical performance of a semi-quantitative assay for SARS-CoV2 IgG and SARS-CoV2 IgM antibodies. Clin Chim Acta 2020; 510: 790-5.
- Imai KT, S.: Ikeda, M.: Noguchi, S.: Kitagawa, Y.: Matuoka, M.: Miyoshi, K.: Tarumoto, N.: Sakai, J.: Ito, T.: Maesaki, S.: Tamura, K.: Maeda, T. Clinical evaluation of an immunochromatographic IgM/IgG antibody assay and chest computed tomography for the diagnosis of COVID-19. J Clin Virol 2020; 128: 104393.
- Qiu XX, Y.: Sun, J.: Wang, X.: Chen, G.: Xu, X.: Liao, S.: Yang, N.: Li, S.: Yang, G.: Tang, Y.: Fan, J.: Xie, W.: Hu, H.: Li, Y.: Zheng, F. Dynamic changes of throat swabs RNA and serum antibodies for SARS-CoV-2 and their diagnostic performances in patients with COVID-19. Emerg Microbes Infect 2020; 9(1): 1974-83.
- Lin YC, Lee YL, Cheng CY, et al. Multicenter evaluation of four immunoassays for the performance of early diagnosis of COVID-19 and assessment of antibody responses of patients with pneumonia in Taiwan. J Microbiol Immunol Infect 2021; 54(5): 816-29.
- Stein DRO, C.: Gretchen, A.: Thorlacius, L.: Fudge, D.: Lang, A.: Sekirov, I.: Morshed, M.: Levett, P. N.: Tran, V.: Kus, J. V.: Gubbay, J.: Mohan, V.: Charlton, C.: Kanji, J. N.: Tipples, G.: Serhir, B.: Therrien, C.: Roger, M.: Jiao, L.: Zahariadis, G.: Needle, R.: Gilbert, L.: Desnoyers, G.: Garceau, R.: Bouhtiauy, I.: Longtin, J.: El-Gabalawy, N.: Dibernardo, A.: Lindsay, L. R.: Drebot, M. Evaluation of commercial SARS-CoV-2 serological assays in Canadian public health laboratories. Diagnostic Microbiology and Infectious Disease 2021; 101(3).
- Suhandynata RT, Hoffman MA, Kelner MJ, McLawhon RW, Reed SL, Fitzgerald RL. Longitudinal Monitoring of SARS-CoV-2 IgM and IgG Seropositivity to Detect COVID-19. J Appl Lab Med 2020; 5(5): 908-20.
- Egger MB, C.: Wiesinger, K.: Gabriel, C.: Clodi, M.: Mueller, T.: Dieplinger, B. Comparison of the Elecsys® Anti-SARS-CoV-2 immunoassay with the EDI™ enzyme linked immunosorbent assays for the detection of SARS-CoV-2 antibodies in human plasma. Clin Chim Acta 2020; 509: 18-21.
- Bundschuh CE, M.: Wiesinger, K.: Gabriel, C.: Clodi, M.: Mueller, T.: Dieplinger, B. Evaluation of the EDI enzyme linked immunosorbent assays for the detection of SARS-CoV-2 IgM and IgG antibodies in human plasma. Clin Chim Acta 2020; 509: 79-82.
- Velay AG, F.: Benotmane, I.: Wendling, M. J.: Danion, F.: Collange, O.: De Sèze, J.: Schmidt-Mutter, C.: Schneider, F.: Bilbault, P.: Meziani, F.: Fafi-Kremer, S. Evaluation of the performance of SARS-CoV-2 serological tools and their positioning in COVID-19 diagnostic strategies. Diagn Microbiol Infect Dis 2020; 98(4): 115181.
- Whitman JDH, J.: Mowery, C. T.: Shy, B. R.: Yu, R.: Yamamoto, T. N.: Rathore, U.: Goldgof, G. M.: Whitty, C.: Woo, J. M.: Gallman, A. E.: Miller, T. E.: Levine, A. G.: Nguyen, D. N.: Bapat, S. P.: Balcerek, J.: Bylsma, S. A.: Lyons, A. M.: Li, S.: Wong, A. W.: Gillis-Buck, E. M.: Steinhart, Z. B.: Lee, Y.: Apathy, R.: Lipke, M. J.: Smith, J. A.: Zheng, T.: Boothby, I. C.: Isaza, E.: Chan, J.: Acenas, D. D., 2nd: Lee, J.: Macrae, T. A.: Kyaw, T. S.: Wu, D.: Ng, D. L.: Gu, W.: York, V. A.: Eskandarian, H. A.: Callaway, P. C.: Warrier, L.: Moreno, M. E.: Levan, J.: Torres, L.: Farrington, L. A.: Loudermilk, R. P.: Koshal, K.: Zorn, K. C.: Garcia-Beltran, W. F.: Yang, D.: Astudillo, M. G.: Bernstein, B. E.: Gelfand, J. A.: Ryan, E. T.: Charles, R. C.: Iafrate, A. J.: Lennerz, J. K.: Miller, S.: Chiu, C. Y.: Stramer, S. L.: Wilson, M. R.: Manglik, A.: Ye, C. J.: Krogan, N. J.: Anderson, M. S.: Cyster, J. G.: Ernst, J. D.: Wu, A. H. B.: Lynch, K. L.: Bern, C.: Hsu, P. D.: Marson, A. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat Biotechnol 2020; 38(10): 1174-83.
- Nilles EJ, Karlson EW, Norman M, et al. Evaluation of Three Commercial and Two Non-Commercial Immunoassays for the Detection of Prior Infection to SARS-CoV-2. J Appl Lab Med 2021; 6(6): 1561-70 %8 Nov 1 %! Evaluation of Three Commercial and Two Non-Commercial Immunoassays for the Detection of Prior Infection to SARS-CoV-2 %@ 2576-9456 (Print) 2475-7241.
- Kulkarni R, Shrivastava S, Patil H, et al. Performance assessment of SARS-CoV-2 IgM & IgG ELISAs in comparison with plaque reduction neutralization test. Indian Journal of Medical Research 2021; 153(5): 658-64.
- Boum Y, Fai KN, Nikolay B, et al. Performance and operational feasibility of antigen and antibody rapid diagnostic tests for COVID-19 in symptomatic and asymptomatic patients in Cameroon: a clinical, prospective, diagnostic accuracy study. The Lancet Infectious Diseases 2021; 21(8): 1089-96.
- Fischer PU, Fischer K, Curtis KC, et al. Evaluation of commercial rapid lateral flow tests, alone or in combination, for SARS-CoV-2 antibody testing. American Journal of Tropical Medicine and Hygiene 2021; 105(2): 378-86.
- Saluzzo FM, P.: Poletti De Chaurand, V.: Quaresima, V.: Cugnata, F.: Di Serio, C.: Macé, A.: De Vos, M.: Sacks, J. A.: Cirillo, D. M. SARS-CoV-2 Antibody Rapid Tests: Valuable Epidemiological Tools in Challenging Settings. Microbiol Spectr 2021: e0025021.
- Gebrecherkos T, Kiros YK, Challa F, et al. Longitudinal profile of antibody response to SARS-CoV-2 in patients with COVID-19 in a setting from Sub-Saharan Africa: A prospective longitudinal study. PLoS One 2022; 17(3): e0263627.
- Pegoraro MM, V.: Salvagno, G. L.: Gaino, S.: Bassi, A.: Caloi, C.: Peretti, A.: Bizzego, S.: Poletto, L.: Bovo, C.: Lippi, G.: Lo Cascio, G. Evaluation of three immunochromatographic tests in COVID-19 serologic diagnosis and their clinical usefulness. European Journal of Clinical Microbiology and Infectious Diseases 2021; 40(4): 897-900.
- Soleimani RK, M.: Gruson, D.: Rodriguez-Villalobos, H.: Berghmans, M.: Belkhir, L.: Yombi, J. C.: Kabamba-Mukadi, B. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID-19 patients. J Med Virol 2021; 93(3): 1465-77.
- Montesinos IG, D.: Kabamba, B.: Dahma, H.: Van den Wijngaert, S.: Reza, S.: Carbone, V.: Vandenberg, O.: Gulbis, B.: Wolff, F.: Rodriguez-Villalobos, H. Evaluation of two automated and three rapid lateral flow immunoassays for the detection of anti-SARS-CoV-2 antibodies. J Clin Virol 2020; 128: 104413.
- Ou JT, M.: He, H.: Tan, H.: Mai, J.: Long, Y.: Jiang, X.: He, Q.: Huang, Y.: Li, Y.: Chen, R.: Li, L.: Shi, Y.: Li, F. SARS-CoV-2 Antibodies and Associated Factors at Different Hospitalization Time Points in 192 COVID-19 Cases. The journal of applied laboratory medicine 2021.
- Al Haddad C, Finianos P, Germanos M, Feghali R, Hasan H. Comparison of four automated SARS-CoV-2 serological immunoassays in Lebanon. Ann Biol Clin (Paris) 2021; 79(6): 579-86.
- Wakita MI, M.: Saito, K.: Horiuchi, Y.: Yamatani, K.: Ishikawa, S.: Yamamoto, T.: Igawa, G.: Hinata, M.: Kadota, K.: Kurosawa, T.: Takahashi, S.: Saito, T.: Misawa, S.: Akazawa, C.: Naito, T.: Miida, T.: Takahashi, K.: Ai, T.: Tabe, Y. Comparison of the clinical performance and usefulness of five SARS-CoV-2 antibody tests. PLoS ONE 2021; 16(2 February).
- Tré-Hardy MW, A.: Beukinga, I.: Favresse, J.: Dogné, J. M.: Douxfils, J.: Blairon, L. Analytical and clinical validation of an ELISA for specific SARS-CoV-2 IgG, IgA, and IgM antibodies. J Med Virol 2021; 93(2): 803-11.
- Jacot DM, M.: Coste, A. T.: Aubry, C.: Sacks, J. A.: Greub, G.: Croxatto, A. Evaluation of sixteen ELISA SARS-CoV-2 serological tests. Journal of Clinical Virology 2021; 142.
- Pecoraro VC, T.: Meacci, M.: Gargiulo, R.: Capobianchi, M. R.: Mussini, C.: Vecchi, E.: Pecorari, M.: Gagliotti, C.: Trenti, T.: Sarti, M. Accuracy of the serological detection of IgG and IgM to SARS-Cov-2: a prospective, cross-sectional study. European Journal of Clinical Microbiology and Infectious Diseases 2021; 40(9): 1891-8.
- Yamamoto M, Okazaki K, Kitai Y, et al. Comparison of six antibody assays and two combination assays for COVID-19. Virol J 2022; 19(1): 24.
- Mairesse AF, J.: Eucher, C.: Elsen, M.: Tré-Hardy, M.: Haventith, C.: Gruson, D.: Dogné, J. M.: Douxfils, J.: Göbbels, P. High clinical performance and quantitative assessment of antibody kinetics using a dual recognition assay for the detection of SARS-CoV-2 IgM and IgG antibodies. Clin Biochem 2020; 86: 23-7.
- Nakano YK, M.: Morita, Y.: Shimura, T.: Yokoyama, R.: Qian, C.: Xia, F.: He, F.: Kishi, Y.: Okada, J.: Yoshikawa, N.: Nagura, Y.: Okazaki, H.: Moriya, K.: Seto, Y.: Kodama, T.: Yatomi, Y. Time course of the sensitivity and specificity of anti-SARS-CoV-2 IgM and IgG antibodies for symptomatic COVID-19 in Japan. Scientific reports 2021; 11(1): 2776.
- Guedez-López GVA-G, M.: González-Donapetry, P.: Bloise, I.: Tornero-Marin, C.: González-García, J.: Mingorance, J.: García-Rodríguez, J.: Montero-Vega, M. D.: Romero, M. P.: García-Bujalance, S.: Cendejas-Bueno, E.: Ruiz-Carrascoso, G.: Lázaro-Perona, F.: Falces-Romero, I.: Gutiérrez-Arroyo, A.: Girón de Velasco-Sada, P.: Rico Nieto, A.: Loeches, B.: Ruiz-Bastián, M.: Gómez-Arroyo, B.: García-Clemente, P.: Liras-Hernández, M. G.: García-Sánchez, C.: Sánchez-Castellano, M.: San José-Villar, S.: Tato, E.: Romero Huertas, C.: Molina Muñoz, E. Evaluation of three immunochromatographic tests for rapid detection of antibodies against SARS-CoV-2. European Journal of Clinical Microbiology and Infectious Diseases 2020; 39(12): 2289-97.
- Chansaenroj JY, R.: Posuwan, N.: Puenpa, J.: Sudhinaraset, N.: Chirathaworn, C.: Poovorawan, Y. Detection of SARS-CoV-2-specific antibodies via rapid diagnostic immunoassays in COVID-19 patients. Virol J 2021; 18(1): 52.
- Choi HW, Jeon CH, Won EJ, Kang SJ, Lee SY, Kee SJ. Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Serological Diagnostic Tests and Antibody Kinetics in Coronavirus Disease 2019 Patients. Front Microbiol 2022; 13: 881038.
- Buntinx FC, P.: Gulikers, M.: Verbakel, J.: Jan, L.: Van der Elst, M.: Van Elslande, J.: Van Ranst, M.: Vermeersch, P. Added value of anti-SARS-CoV-2 antibody testing in a Flemish nursing home during an acute COVID-19 outbreak in April 2020. Acta Clin Belg 2020: 1-6.
- Wolff FD, H.: Duterme, C.: Van den Wijngaert, S.: Vandenberg, O.: Cotton, F.: Montesinos, I. Monitoring antibody response following SARS-CoV-2 infection: diagnostic efficiency of 4 automated immunoassays. Diagn Microbiol Infect Dis 2020; 98(3): 115140.
- Wolf JK, T.: Pehnke, S.: Nickel, O.: Lübbert, C.: Kalbitz, S.: Arnold, B.: Ermisch, J.: Berger, L.: Schroth, S.: Isermann, B.: Borte, S.: Biemann, R. Differences of SARS-CoV-2 serological test performance between hospitalized and outpatient COVID-19 cases. Clinica Chimica Acta 2020; 511: 352-9.
- Zervou FN, Louie P, Stachel A, et al. SARS-CoV-2 antibodies: IgA correlates with severity of disease in early COVID-19 infection. Journal of Medical Virology 2021; 93(9): 5409-15.
- Bal AP, B.: Trabaud, M. A.: Escuret, V.: Rabilloud, M.: Langlois-Jacques, C.: Paul, A.: Guibert, N.: D'Aubarède-Frieh, C.: Massardier-Pilonchery, A.: Fabien, N.: Goncalves, D.: Boibieux, A.: Morfin-Sherpa, F.: Pitiot, V.: Gueyffier, F.: Lina, B.: Fassier, J. B.: Trouillet-Assant, S. Evaluation of High-Throughput SARS-CoV-2 Serological Assays in a Longitudinal Cohort of Patients with Mild COVID-19: Clinical Sensitivity, Specificity, and Association with Virus Neutralization Test. Clinical chemistry 2021; 67(5): 742-52.
- Traugott MA, S. W.: Aberle, J. H.: Griebler, H.: Karolyi, M.: Pawelka, E.: Puchhammer-Stöckl, E.: Zoufaly, A.: Weseslindtner, L. Performance of Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Assays in Different Stages of Infection: Comparison of Commercial Enzyme-Linked Immunosorbent Assays and Rapid Tests. J Infect Dis 2020; 222(3): 362-6.
- Nicholson SK, T.: Khvorov, A.: Douros, C.: Mordant, F.: Bond, K.: Druce, J.: Williamson, D. A.: Purcell, D.: Lewin, S. R.: Sullivan, S.: Subbarao, K.: Catton, M. Evaluation of 6 Commercial SARS-CoV-2 Serology Assays Detecting Different Antibodies for Clinical Testing and Serosurveillance. Open Forum Infectious Diseases 2021; 8(7).
- Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur Respir J 2020; 56(2): 2000763.
- Liu Z-L, Liu Y, Wan L-G, et al. Antibody profiles in mild and severe cases of COVID-19. Clin Chem 2020.
- Butterfield TRB-M, A.: Phillips, Y. Z. R.: Brown, N.: Francis, K.: Brown, J.: Walker, J. P.: McKnight, N. A. L.: Ehikhametalor, K.: Taylor, D. K.: Bruce, C. A.: McGrowder, D.: Wharfe, G.: Sandiford, S. L.: Thompson, T. K.: Anzinger, J. J. Assessment of commercial SARS-CoV-2 antibody assays, Jamaica. International Journal of Infectious Diseases 2021; 105: 333-6.
- Dellière SS, M.: Minier, M.: Gabassi, A.: Alanio, A.: Le Goff, J.: Delaugerre, C.: Chaix, M. L. Evaluation of the COVID-19 IgG/IgM Rapid Test from Orient Gene Biotech. J Clin Microbiol 2020; 58(8).
- Dortet LE, C.: Vauloup-Fellous, C.: Khecharem, M.: Ronat, J. B.: Fortineau, N.: Roque-Afonso, A. M.: Naas, T. Rapid Determination of SARS-CoV-2 antibodies using a bedside, point-of-Care, serological test. Emerg Microbes Infect 2020; 9(1): 2212-21.
- Ali AH, Zidan AS. Detection of Antibodies in Patients with COVID-19 by Rapid Chromatographic Immunoassay. Turkish Journal of Immunology 2022; 10(2): 115-20.
- Serre-Miranda CN, C.: Roque, S.: Canto-Gomes, J.: Silva, C. S.: Vieira, N.: Barreira-Silva, P.: Alves-Peixoto, P.: Cotter, J.: Reis, A.: Formigo, M.: Sarmento, H.: Pires, O.: Carvalho, A.: Petrovykh, D. Y.: Diéguez, L.: Sousa, J. C.: Sousa, N.: Capela, C.: Palha, J. A.: Cunha, P. G.: Correia-Neves, M. Performance assessment of 11 commercial serological tests for SARS-CoV-2 on hospitalised COVID-19 patients. International Journal of Infectious Diseases 2021; 104: 661-9.
- Infantino MG, V.: Lari, B.: Bambi, R.: Perri, A.: Manneschi, M.: Terenzi, G.: Liotti, I.: Ciotta, G.: Taddei, C.: Benucci, M.: Casprini, P.: Veneziani, F.: Fabbri, S.: Pompetti, A.: Manfredi, M. Diagnostic accuracy of an automated chemiluminescent immunoassay for anti-SARS-CoV-2 IgM and IgG antibodies: an Italian experience. J Med Virol 2020; 92(9): 1671-5.
- Turbett SEA, M.: Dighe, A. S.: Garcia Beltran, W.: Miller, T.: Scott, H.: Durbin, S. M.: Bharadwaj, M.: Thomas, J.: Gogakos, T. S.: Astudillo, M.: Lennerz, J.: Rosenberg, E. S.: Branda, J. A. Evaluation of Three Commercial SARS-CoV-2 Serologic Assays and Their Performance in Two-Test Algorithms. J Clin Microbiol 2020; 59(1).
- Harley KG, I. L. Comparison of the Clinical Performances of the Abbott Alinity IgG, Abbott Architect IgM, and Roche Elecsys Total SARS-CoV-2 Antibody Assays. J Clin Microbiol 2020; 59(1).
- Paiva KJG, R. D.: Chan, P. A.: Huard, R. C.: Caliendo, A. M.: Lonks, J. R.: King, E.: Tang, E. W.: Pytel-Parenteau, D. L.: Nam, G. H.: Yakirevich, E.: Lu, S. Validation and performance comparison of three SARS-CoV-2 antibody assays. J Med Virol 2021; 93(2): 916-23.
- Tang MSH, K. G.: Logsdon, N. M.: Hayes, J. E.: Gronowski, A. M.: Anderson, N. W.: Farnsworth, C. W. Clinical Performance of Two SARS-CoV-2 Serologic Assays. Clin Chem 2020; 66(8): 1055-62.
- Chew KLT, S. S.: Saw, S.: Pajarillaga, A.: Zaine, S.: Khoo, C.: Wang, W.: Tambyah, P.: Jureen, R.: Sethi, S. K. Clinical evaluation of serological IgG antibody response on the Abbott Architect for established SARS-CoV-2 infection. Clin Microbiol Infect 2020; 26(9): 1256.e9-.e11.
- Tan SSS, S.: Chew, K. L.: Huak, C. Y.: Khoo, C.: Pajarillaga, A.: Wang, W.: Tambyah, P.: Ong, L.: Jureen, R.: Sethi, S. K. Head-to-head evaluation on diagnostic accuracies of six SARS-CoV-2 serological assays. Pathology 2020; 52(7): 770-7.
- Theel ESH, J.: Hilgart, H.: Granger, D. Performance Characteristics of Four High-Throughput Immunoassays for Detection of IgG Antibodies against SARS-CoV-2. J Clin Microbiol 2020; 58(8).
- Aoki KT, K.: Nagasawa, T.: Kashiwagi, K.: Mori, N.: Matsubayashi, K.: Satake, M.: Tanaka, I.: Kodama, N.: Shimodaira, T.: Ishii, Y.: Miyazaki, T.: Ishii, T.: Morita, T.: Yoshimura, T.: Tateda, K. Combination of a SARS-CoV-2 IgG Assay and RT-PCR for Improved COVID-19 Diagnosis. Ann Lab Med 2021; 41(6): 568-76.
- Wehrhahn MCB, S. J.: Newcombe, J. P.: Chong, S.: Evans, J.: Figtree, M.: Hainke, L.: Hueston, L.: Khan, S.: Marland, E.: O'Sullivan, M. V. N.: Powell, H.: Roy, J.: Waring, L.: Yu, M.: Robson, J. An evaluation of 4 commercial assays for the detection of SARS-CoV-2 antibodies in a predominantly mildly symptomatic low prevalence Australian population. Journal of Clinical Virology 2021; 138.
- Hubbard JAG, K. A.: Khan, J.: Szczepiorkowski, Z. M.: de Gijsel, D.: Ovalle, A. A.: AlSalman, A. S.: Gallagher, T. L.: Johnston, A. A.: Tibbetts, A. R.: Vital, S. E.: Cervinski, M. A.: Nerenz, R. D. Comparison of Two Automated Immunoassays for the Detection of SARS-CoV-2 Nucleocapsid Antibodies. The journal of applied laboratory medicine 2021; 6(2): 429-40.
- Therrien CS, B.: Bélanger-Collard, M.: Skrzypczak, J.: Shank, D. K.: Renaud, C.: Girouard, J.: Loungnarath, V.: Carrier, M.: Brochu, G.: Tourangeau, F.: Gilfix, B.: Piche, A.: Bazin, R.: Guérin, R.: Lavoie, M.: Martel-Laferrière, V.: Fortin, C.: Benoit, A.: Marcoux, D.: Gauthier, N.: Laumaea, A. M.: Gasser, R.: Finzi, A.: Roger, M. Multicenter evaluation of the clinical performance and the neutralizing antibody activity prediction properties of 10 high-throughput serological assays used in clinical laboratories. Journal of Clinical Microbiology 2021; 59(3).
- Bond KAW, E.: Nicholson, S.: Lim, S.: Johnson, D.: Cox, B.: Putland, M.: Gardiner, E.: Tippett, E.: Graham, M.: Mordant, F.: Catton, M.: Lewin, S. R.: Subbarao, K.: Howden, B. P.: Williamson, D. A. Longitudinal evaluation of laboratory-based serological assays for SARS-CoV-2 antibody detection. Pathology 2021; 53(6): 773-9.
- Maine GN, Krishnan SM, Walewski K, Trueman J, Sykes E, Sun Q. Clinical and analytical evaluation of the Abbott AdviseDx quantitative SARS-CoV-2 IgG assay and comparison with two other serological tests. J Immunol Methods 2022; 503: 113243.
- Chen SYL, Y. L.: Lin, Y. C.: Lee, N. Y.: Liao, C. H.: Hung, Y. P.: Lu, M. C.: Wu, J. L.: Tseng, W. P.: Lin, C. H.: Chung, M. Y.: Kang, C. M.: Lee, Y. F.: Lee, T. F.: Cheng, C. Y.: Chen, C. P.: Huang, C. H.: Liu, C. E.: Cheng, S. H.: Ko, W. C.: Hsueh, P. R.: Chen, S. C. Multicenter evaluation of two chemiluminescence and three lateral flow immunoassays for the diagnosis of COVID-19 and assessment of antibody dynamic responses to SARS-CoV-2 in Taiwan. Emerg Microbes Infect 2020; 9(1): 2157-68.
- Plaga AW, R.: Olson, E.: Payto, D.: Harrington, J.: Nwe-Kissig, P. T.: Strizzi, M.: Zilka, S.: Ko, J.: Colón-Franco, J. M. Evaluation of the Clinical Performance of 7 Serological Assays for SARS-CoV-2 for Use in Clinical Laboratories. The journal of applied laboratory medicine 2021; 6(4): 998-1004.
- Catry EJ, H.: Dodemont, M.: Saad Albichr, I.: Lardinois, B.: de fays, B.: Delaere, B.: Closset, M.: Laurent, T.: Denis, O.: Galanti, L.: Mullier, F.: Huang, T. D. Analytical and clinical evaluation of four commercial SARS-CoV-2 serological immunoassays in hospitalized patients and ambulatory individuals. Journal of Virological Methods 2021; 289.
- Harrington DA, T.: Rosmarin, C.: Cutino-Moguel, T.: Hopkins, M. Evaluation of 2 commercial anti-SARS-CoV-2 antibody assays in an immunocompetent and immunocompromised inpatient population with COVID-19. Diagn Microbiol Infect Dis 2021; 101(2): 115449.
- Yun SR, J. H.: Jang, J. H.: Bae, H.: Yoo, S. H.: Choi, A. R.: Jo, S. J.: Lim, J.: Lee, J.: Ryu, H.: Cho, S. Y.: Lee, D. G.: Lee, J.: Kim, S. C.: Park, Y. J.: Lee, H.: Oh, E. J. Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays. Annals of laboratory medicine 2021; 41(6): 577-87.
- Yang JP, E. C.: Hamilton, C.: Neibauer, T.: Robyak, K.: McGhee, P.: Speicher, T.: Zhu, Y. Analytical and Clinical Analysis of Two Automated Anti-SARS-CoV-2 Immunoassays in Pre-Pandemic and Pandemic Patient Populations. The journal of applied laboratory medicine 2021; 6(2): 441-50.
- Bryan AP, G.: Wener, M. H.: Fink, S. L.: Morishima, C.: Chaudhary, A.: Jerome, K. R.: Mathias, P. C.: Greninger, A. L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J Clin Microbiol 2020; 58(8).
- Lokida D, Karyana M, Kosasih H, et al. Performance and correlation of ten commercial immunoassays for the detection of SARS-CoV-2 antibodies. Heliyon 2022; 8(12): e12614.
- Escribano PÁ-U, A.: Alonso, R.: Catalán, P.: Alcalá, L.: Muñoz, P.: Guinea, J. Detection of SARS-CoV-2 antibodies is insufficient for the diagnosis of active or cured COVID-19. Sci Rep 2020; 10(1): 19893.
- Kohmer NW, S.: Rühl, C.: Ciesek, S.: Rabenau, H. F. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol 2020; 129: 104480.
- Kubota KK, Y.: Matsuoka, M.: Imai, K.: Orihara, Y.: Kawamura, R.: Sakai, J.: Ishibashi, N.: Tarumoto, N.: Takeuchi, S.: Maesaki, S.: Maeda, T. Clinical evaluation of the antibody response in patients with COVID-19 using automated high-throughput immunoassays. Diagnostic Microbiology and Infectious Disease 2021; 100(3).
- Jugwanth S, Gededzha MP, Mampeule N, et al. Performance of the Abbott SARS-CoV-2 IgG serological assay in South African 2 patients. PLoS ONE 2022; 17(2): e0262442.
- Merrill AEJ, J. B.: Ehlers, A.: Voss, D.: Krasowski, M. D. Head-to-Head Comparison of Two SARS-CoV-2 Serology Assays. J Appl Lab Med 2020; 5(6): 1351-7.
- Huyghe EJ, H.: Matheeussen, V.: Hoffbauer, I.: Goossens, H.: Peeters, B. Performance of three automated SARS-CoV-2 antibody assays and relevance of orthogonal testing algorithms. Clin Chem Lab Med 2020; 59(2): 411-9.
- Kittel MM, M. C.: Zahn, I.: Roth, H. J.: Thiaucourt, M.: Gerhards, C.: Haselmann, V.: Neumaier, M.: Findeisen, P. Clinical evaluation of commercial automated SARS-CoV-2 immunoassays. Int J Infect Dis 2021; 103: 590-6.
- Nilsson ACH, D. K.: Justesen, U. S.: Gorm-Jensen, T.: Andersen, N. S.: Øvrehus, A.: Johansen, I. S.: Michelsen, J.: Sprogøe, U.: Lillevang, S. T. Comparison of six commercially available SARS-CoV-2 antibody assays-Choice of assay depends on intended use. Int J Infect Dis 2021; 103: 381-8.
- Caturegli GM, J.: Howard, B. M.: Caturegli, P. Clinical Validity of Serum Antibodies to SARS-CoV-2 : A Case-Control Study. Ann Intern Med 2020; 173(8): 614-22.
- Cota GF, M. L.: de Souza, C. S.: Pedras, M. J.: Saliba, J. W.: Faria, V.: Alves, L. L.: Rabello, A.: Avelar, D. M. Diagnostic performance of commercially available COVID-19 serology tests in Brazil. International Journal of Infectious Diseases 2020; 101: 382-90.
- Buchholtz MLA, F. M.: Eichhorn, P.: Weigand, M.: Kleinhempel, A.: Häusler, K.: Bruegel, M.: Holdt, L. M.: Teupser, D. SARS-CoV-2 antibody immunoassays in serial samples reveal earlier seroconversion in acutely ill COVID-19 patients developing ARDS. PLoS ONE 2021; 16(5 May).
- Favresse JC, J.: Eucher, C.: Elsen, M.: Laffineur, K.: Dogné, J. M.: Douxfils, J. Clinical performance of three fully automated anti-SARS-CoV-2 immunoassays targeting the nucleocapsid or spike proteins. Journal of Medical Virology 2021; 93(4): 2262-9.
- Van Elslande JH, E.: Depypere, M.: Brackenier, A.: Desmet, S.: André, E.: Van Ranst, M.: Lagrou, K.: Vermeersch, P. Diagnostic performance of seven rapid IgG/IgM antibody tests and the Euroimmun IgA/IgG ELISA in COVID-19 patients. Clin Microbiol Infect 2020; 26(8): 1082-7.
- Huber TS, P.: Irrgang, P.: Korn, K.: Tenbusch, M.: Diesch, K.: Achenbach, S.: Kremer, A. E.: Werblow, M.: Vetter, M.: Bogdan, C.: Held, J. Diagnostic performance of four SARS-CoV-2 antibody assays in patients with COVID-19 or with bacterial and non-SARS-CoV-2 viral respiratory infections. European Journal of Clinical Microbiology and Infectious Diseases 2021.
- Hörber SS, J.: Relker, L.: Jürgens, S.: Guther, J.: Peter, S.: Lehmann, R.: Peter, A. Evaluation of three fully-automated SARS-CoV-2 antibody assays. Clinical Chemistry and Laboratory Medicine 2020; 58(12): 2113-20.
- Sarina Yang H, Racine-Brzostek SE, Lee WT, et al. SARS-CoV-2 antibody characterization in emergency department, hospitalized and convalescent patients by two semi-quantitative immunoassays. Clin Chim Acta 2020.
- Yassine HMA-J, H.: Al-Sadeq, D. W.: Dargham, S. R.: Younes, S. N.: Shurrab, F.: Marei, R. M.: Hssain, A. A.: Taleb, S.: Alhussain, H.: Al-Nesf, M. A.: Al-Khal, A.: Qotba, H.: Althani, A. A.: Tang, P.: Abu-Raddad, L. J.: Nasrallah, G. K. Performance evaluation of five ELISA kits for detecting anti-SARS-COV-2 IgG antibodies. International Journal of Infectious Diseases 2021; 102: 181-7.
- Florin LM, K.: Vandewal, W.: Bernard, D.: Robbrecht, J. Performance Evaluation of the Siemens SARS-CoV-2 Total Antibody and IgG Antibody Test. Laboratory medicine 2021.
- Igawa G, Yamamoto T, Baba Y, et al. Clinical Evaluation of Siemens SARS-CoV-2 Total Antibody assay and IgG assay using the Dimension EXL 200 in the Tokyo Metropolitan area. Heliyon 2021; 7(11): e08393 %7 20211116 %8 Nov %! Clinical Evaluation of Siemens SARS-CoV-2 Total Antibody assay and IgG assay using the Dimension EXL 200 in the Tokyo Metropolitan area %@ 2405-8440 (Print) 2405-8440.
- Tan SSS, S.: Chew, K. L.: Wang, C.: Pajarillaga, A.: Khoo, C.: Wang, W.: Ali, Z. M.: Yang, Z.: Chan, Y. H.: Tambyah, P.: Jureen, R.: Sethi, S. K. Comparative Clinical Evaluation of the Roche Elecsys and Abbott Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Serology Assays for Coronavirus Disease 2019 (COVID-19). Arch Pathol Lab Med 2021; 145(1): 32-8.
- Charpentier CI, H.: Damond, F.: Bouvet, E.: Chaix, M. L.: Ferré, V.: Delaugerre, C.: Mahjoub, N.: Larrouy, L.: Le Hingrat, Q.: Visseaux, B.: Mackiewicz, V.: Descamps, D.: Fidouh-Houhou, N. Performance evaluation of two SARS-CoV-2 IgG/IgM rapid tests (Covid-Presto and NG-Test) and one IgG automated immunoassay (Abbott). J Clin Virol 2020; 132: 104618.
- Marlet JP, C.: Ragot, E.: Abou El Fattah, Y.: Guillon, A.: Marchand Adam, S.: Lemaignen, A.: Bernard, L.: Desoubeaux, G.: Blasco, H.: Barin, F.: Stefic, K.: Gaudy-Graffin, C. Clinical performance of four immunoassays for antibodies to SARS-CoV-2, including a prospective analysis for the diagnosis of COVID-19 in a real-life routine care setting. J Clin Virol 2020; 132: 104633.
- Tanis JV, E.: Piérard, D.: Weets, I.: Bjerke, M.: Schiettecatte, J.: De Geyter, D. Evaluation of four laboratory-based SARS-CoV-2 IgG antibody immunoassays. Diagnostic Microbiology and Infectious Disease 2021; 100(1).
- Vauloup-Fellous CM, S.: Périllaud-Dubois, C.: Brichler, S.: Alloui, C.: Gordien, E.: Rameix-Welti, M. A.: Gault, E.: Moreau, F.: Fourati, S.: Challine, D.: Pawlotsky, J. M.: Houhou-Fidouh, N.: Damond, F.: Mackiewicz, V.: Charpentier, C.: Méritet, J. F.: Rozenberg, F.: Podglajen, I.: Marot, S.: Petit, H.: Burrel, S.: Akhavan, S.: Leruez-Ville, M.: Avettand-Fenoel, V.: Fourgeaud, J.: Guilleminot, T.: Gardiennet, E.: Bonacorsi, S.: Carol, A.: Carcelain, G.: Villemonteix, J.: Boukli, N.: Gozlan, J.: Morand-Joubert, L.: Legoff, J.: Delaugerre, C.: Chaix, M. L.: Roque-Afonso, A. M.: Dortet, L.: Naas, T.: Ronat, J. B.: Lepape, S.: Marcelin, A. G.: Descamps, D. Performance of 30 commercial SARS-CoV-2 serology assays in testing symptomatic COVID-19 patients. Eur J Clin Microbiol Infect Dis 2021; 40(10): 2235-41.
- Prazuck TC, M.: Giachè, S.: Gubavu, C.: Seve, A.: Rzepecki, V.: Chevereau-Choquet, M.: Kiani, C.: Rodot, V.: Lionnet, E.: Courtellemont, L.: Guinard, J.: Pialoux, G.: Hocqueloux, L. Evaluation of performance of two SARS-CoV-2 Rapid IgM-IgG combined antibody tests on capillary whole blood samples from the fingertip. PLoS One 2020; 15(9): e0237694.
- Sisay AT, A.: Desale, A.: Ataro, I.: Woldesenbet, Z.: Nigusse, B.: Tayachew, A.: Kebede, A.: Desta, A. F. Diagnostic Performance of SARS-CoV-2 IgM/IgG Rapid Test Kits for the Detection of the Novel Coronavirus in Ethiopia. J Multidiscip Healthc 2021; 14: 171-80.
- Puschel KF, C.: Peñaloza, B.: Abarca, K.: Rojas, M. P.: Tellez, A.: Moore, P.: Cea, A. M.: Wilson, C.: Cid, V.: Montero, J. Clinical and serological profile of asymptomatic and non-severe symptomatic COVID-19 cases: Lessons from a longitudinal study in primary care in Latin America. BJGP Open 2021; 5(1): 1-8.
- Ong DSYdM, S. J.: Lindeboom, F. A.: Koeleman, J. G. M. Comparison of diagnostic accuracies of rapid serological tests and ELISA to molecular diagnostics in patients with suspected coronavirus disease 2019 presenting to the hospital. Clin Microbiol Infect 2020; 26(8): 1094.e7-.e10.
- Shen B, Zheng Y, Zhang X, et al. Clinical evaluation of a rapid colloidal gold immunochromatography assay for SARS-Cov-2 IgM/IgG. Am J Transl Res 2020; 12(4): 1348-54.
- Rostamzadeh DM, S.: Alinejad, M.: Jooya, S. R.: Eskandarian, M.: Metvaei, A.: Vafaei, S.: Aboulghasemi, H.: Younesi, V.: Shabani, M. Serological assay for anti-SARS-CoV-2 antibodies improves sensitivity of diagnosis of COVID-19 patients. Medical Microbiology and Immunology 2021.
- Kim JK, Ryu SW, Kim JS, Jung BK. Performance evaluation of four rapid antibody tests for the detection of severe acute respiratory syndrome coronavirus 2. Journal of Clinical Laboratory Analysis 2022.
- Ozturk AB, T.: Simsek Bozok, T. Evaluation of rapid antibody test and chest computed tomography results of COVID-19 patients: A retrospective study. Journal of Medical Virology 2021.
- Pérez-García FP-T, R.: Iglesias, M. E.: Romanyk, J.: Arroyo, T.: Gómez-Herruz, P.: González, R.: Lapeña García, S.: Cuadros-González, J. Comparative evaluation of six immunoassays for the detection of antibodies against SARS-CoV-2. Journal of Virological Methods 2021; 289.
- Pallett SJCD, S. J.: Patel, A.: Charani, E.: Mughal, N.: Stebbing, J.: Davies, G. W.: Moore, L. S. P. Point-of-care SARS-CoV-2 serological assays for enhanced case finding in a UK inpatient population. Sci Rep 2021; 11(1): 5860.
- Baron RCR, L.: Weber, M.: Thiel, S.: Grossmann, K.: Wohlwend, N.: Lung, T.: Hillmann, D.: Ritzler, M.: Bigler, S.: Egli, K.: Ferrara, F.: Bodmer, T.: Imperiali, M.: Heer, S.: Renz, H.: Flatz, L.: Kohler, P.: Vernazza, P.: Kahlert, C. R.: Paprotny, M.: Risch, M. Frequency of serological non-responders and false-negative RT-PCR results in SARS-CoV-2 testing: A population-based study. Clinical Chemistry and Laboratory Medicine 2020; 58(12): 2131-40.
- Chamkhi S, Dhaouadi T, Sfar I, et al. Comparative study of six SARS-CoV-2 serology assays: Diagnostic performance and antibody dynamics in a cohort of hospitalized patients for moderate to critical COVID-19. Int J Immunopathol Pharmacol 2022; 36: 20587384211073232.
- Charpentier CP, G.: Ichou, H.: Ferré, V. M.: Larfi, I.: Phung, B. C.: Vallois, D.: LeGac, S.: Aubier, M.: Descamps, D.: Fidouh-Houhou, N.: Bouvet, E. Contribution of rapid lateral flow assays from capillary blood specimens to the diagnosis of COVID-19 in symptomatic healthcare workers: a pilot study in a university hospital, Paris, France. Diagn Microbiol Infect Dis 2021; 101(2): 115430.
- Chen JN, Z.: Li, H.: Tang, D.: Zhang, P. Analysis of Nucleic Acid and Antibody Detection Results for SARS-CoV-2 Infection. Arch Iran Med 2021; 24(5): 427-33.
- Cross GB, Naftalin CM, Ngiam JN, et al. Discrepant serological findings in SARS-CoV-2 PCR-negative hospitalized patients with fever and acute respiratory symptoms during the pandemic. Journal of Medical Virology 2022.
- de Almeida SMS, R. N.: Nogueira, M. B.: Sanada, B.: Cavalli, B. M.: Rotta, I.: Takahashi, G. R. A.: Pereira, L. A.: Moreira, F. B.: Dino, C. L. T.: Graf, M. E.: de Almeida, B. M. M.: Domino, N. R.: Genelhoud, G.: Bochnia-Bueno, L.: Raboni, S. M. Rapid Serological Tests for SARS-CoV-2: Diagnostic Performance of 4 Commercial Assays. Med Princ Pract 2021; 30(4): 385-94.
- Mirijello AZ, M.: Miscio, G.: de Matthaeis, A.: Piscitelli, P.: Carbonelli, C. M.: Di Giorgio, A.: Inglese, M.: Ciliberti, G. L.: Marciano, C.: Borelli, C.: Vergara, D.: Castorani, G.: Orciulo, G. V.: Di Mauro, L.: Carella, M.: Simeone, A.: Copetti, M.: Leone, M. A.: De Cosmo, S. Diagnosis of covid-19 in patients with negative nasopharyngeal swabs: Reliability of radiological and clinical diagnosis and accuracy versus serology. Diagnostics 2021; 11(3).
- Nakagama YK, Y.: Candray, K.: Nakagama, S.: Sano, F.: Tsuchida, T.: Kunishima, H.: Imai, T.: Shintani, A.: Nitahara, Y.: Kaku, N.: Kido, Y. Serological Testing Reveals the Hidden COVID-19 Burden among Health Care Workers Experiencing a SARS-CoV-2 Nosocomial Outbreak. Microbiol Spectr 2021: e0108221.
- Pan Y, Li X, Yang G, et al. Serological immunochromatographic approach in diagnosis with SARS-CoV-2 infected COVID-19 patients. J Infect 2020; 81(1): e28-e32.
- Reich N, Lowe CF, Puddicombe D, et al. Repeat virological and serological profiles in hospitalized patients initially tested by nasopharyngeal RT-PCR for SARS-CoV-2. J Med Virol 2021; 93(12): 6808-12.
- Rostamzadeh D, Mortezagholi S, Alinejad M, et al. Serological assay for anti-SARS-CoV-2 antibodies improves sensitivity of diagnosis of COVID-19 patients. Med Microbiol Immunol 2021; 210(5-6): 283-9.
- Sacristan MSC-B, A.: Cintas, M. I. Z.: García, A. S.: de Villavicencio, C. Y.: Maestre, M. M. Comparison of various serological assays for novel SARS-COV-2. Eur J Clin Microbiol Infect Dis 2021; 40(5): 963-8.
- Tawiah KDH, K. G.: Farnsworth, C. W. Limited Diagnostic Utility of SARS-CoV-2 Serologic Testing in Symptomatic PCR Negative Patients. J Appl Lab Med 2021; 6(4): 1083-5.
- Van Praet JTC, A. S.: Van De Moortele, K.: Descheemaeker, P.: Reynders, M. Comparison of four commercial SARS-CoV-2 IgG immuno-assays in RT-PCR negative patients with suspect CT findings. Infection 2021; 49(1): 145-8.
- Yokoyama RK, M.: Morita, Y.: Shimura, T.: Nakano, Y.: Qian, C.: Xia, F.: He, F.: Kishi, Y.: Okada, J.: Yoshikawa, N.: Nagura, Y.: Okazaki, H.: Moriya, K.: Seto, Y.: Kodama, T.: Yatomi, Y. Validation of a new automated chemiluminescent anti-SARS-CoV-2 IgM and IgG antibody assay system detecting both N and S proteins in Japan. PLoS One 2021; 16(3): e0247711.
- Zhao JY, Q.: Wang, H.: Liu, W.: Liao, X.: Su, Y.: Wang, X.: Yuan, J.: Li, T.: Li, J.: Qian, S.: Hong, C.: Wang, F.: Liu, Y.: Wang, Z.: He, Q.: Li, Z.: He, B.: Zhang, T.: Fu, Y.: Ge, S.: Liu, L.: Zhang, J.: Xia, N.: Zhang, Z. Antibody Responses to SARS-CoV-2 in Patients with Novel Coronavirus Disease 2019. Clinical Infectious Diseases 2020; 71(16): 2027-34.
- Belhadjer Z, Méot M, Bajolle F, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020; 142(5): 429-36.
- Dufort EM, Koumans EH, Chow EJ, et al. Multisystem Inflammatory Syndrome in Children in New York State. New England Journal of Medicine 2020; 383(4): 347-58.
- Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. New England Journal of Medicine 2020; 383(4): 334-46.
- Grimaud M, Starck J, Levy M, et al. Acute myocarditis and multisystem inflammatory emerging disease following SARS-CoV-2 infection in critically ill children. Annals of Intensive Care 2020; 10(1): 69.
- Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. The Lancet 2020; 395(10237): 1607-8.
- Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020; 369: m2094.
- Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet 2020; 395(10239): 1771-8.
- Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020; 324(3): 259-69.
- Mamishi SE, G. G.: Mohammadi, M.: Abdolsalehi, M. R.: Sadeghi, R. H.: Mahmoudi, S.: Pourakbari, B. Detection of SARS-CoV-2 antibodies in pediatric patients: An Iranian referral hospital-based study. Human Antibodies 2021; 29(3): 217-23.
- Sweeney NM, B.: Pedro Galão, R.: Pickering, S.: Botgros, A.: Wilson, H. D.: Signell, A. W.: Betancor, G.: Tan, M. K. I.: Ramble, J.: Kouphou, N.: Acors, S.: Graham, C.: Seow, J.: MacMahon, E.: Neil, S. J. D.: Malim, M. H.: Doores, K.: Douthwaite, S.: Batra, R.: Nebbia, G.: Edgeworth, J. D. Clinical utility of targeted SARS-CoV-2 serology testing to aid the diagnosis and management of suspected missed, late or post-COVID-19 infection syndromes: Results from a pilot service implemented during the first pandemic wave. PLoS One 2021; 16(4): e0249791.
- Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Case Definition for MIS-C Available at: https://www.cdc.gov/mis/mis-c/hcp_cstecdc/index.html#:~:text=Case%20Definition%20for%20MIS%2DC&text=Any%20illness%20in%20a%20person,The%20vital%20records%20criteria%20(Suspect) Accessed 8 August 2023.
- Centers for Disease Control and Prevention. Nationwide Commercial Lab Pediatric Antibody Seroprevalence. Available at: https://covid.cdc.gov/covid-data-tracker/#pediatric-seroprevalence. Accessed 10 May 2023.
- McCrindle BW, Harahsheh AS, Handoko R, et al. SARS-CoV-2 Variants and Multisystem Inflammatory Syndrome in Children. N Engl J Med 2023; 388(17): 1624-6.
- Jain E, Donowitz JR, Aarons E, Marshall BC, Miller MP. Multisystem Inflammatory Syndrome in Children after SARS-CoV-2 Vaccination. Emerg Infect Dis 2022; 28(5): 990-3.
- Mallon PWGT, W.: Leon, A. G.: McCann, K.: Kenny, G.: McGettrick, P.: Green, S.: Inzitari, R.: Cottere, A. G.: Feeney, E. R.: Savinelli, S.: Doran, P. Dynamic Change and Clinical Relevance of Postinfectious SARS-CoV-2 Antibody Responses. Open Forum Infect Dis 2021; 8(8): ofab122.
- Charlton CLK, J. N.: Johal, K.: Bailey, A.: Plitt, S. S.: MacDonald, C.: Kunst, A.: Buss, E.: Burnes, L. E.: Fonseca, K.: Berenger, B. M.: Schnabl, K.: Hu, J.: Stokes, W.: Zelyas, N.: Tipples, G. Evaluation of Six Commercial Mid- to High-Volume Antibody and Six Point-of-Care Lateral Flow Assays for Detection of SARS-CoV-2 Antibodies. J Clin Microbiol 2020; 58(10).
- Choe JYK, J. W.: Kwon, H. H.: Hong, H. L.: Jung, C. Y.: Jeon, C. H.: Park, E. J.: Kim, S. K. Diagnostic performance of immunochromatography assay for rapid detection of IgM and IgG in coronavirus disease 2019. J Med Virol 2020; 92(11): 2567-72.
- Meng QBP, J. J.: Wei, X.: Yang, J. Y.: Li, P. C.: Qu, Z. W.: Xiong, Y. F.: Wu, G. J.: Hu, Z. M.: Yu, J. C.: Su, W. Clinical application of combined detection of SARS-CoV-2-specific antibody and nucleic acid. World J Clin Cases 2020; 8(19): 4360-9.
- Manalac JY, J.: Calayag, K.: Nguyen, L.: Patel, P. M.: Zhou, D.: Shi, R. Z. Evaluation of Abbott anti-SARS-CoV-2 CMIA IgG and Euroimmun ELISA IgG/IgA assays in a clinical lab. Clin Chim Acta 2020; 510: 687-90.
- Martinaud CH, C.: Igert, A.: Bigaillon, C.: Bonnet, C.: Mérens, A.: Wolf, A.: Foissaud, V.: Leparc-Goffart, I. Evaluation of the Quotient® MosaiQ™ COVID-19 antibody microarray for the detection of IgG and IgM antibodies to SARS-CoV-2 virus in humans. J Clin Virol 2020; 130: 104571.
- Cobb BL, Lloyd M, Hock KG, Farnsworth CW. Clinical Performance of a Lateral Flow SARS-CoV-2 Total Antibody Assay. J Appl Lab Med 2022; 7(4): 827-33.
- Van Elslande J, Oyaert M, Lorent N, et al. Lower persistence of anti-nucleocapsid compared to anti-spike antibodies up to one year after SARS-CoV-2 infection. Diagn Microbiol Infect Dis 2022; 103(1): 115659.
- Grossberg AN, Koza LA, Ledreux A, et al. A multiplex chemiluminescent immunoassay for serological profiling of COVID-19-positive symptomatic and asymptomatic patients. Nature Communications 2021; 12(1).
- Şener B, Kirbaş E, Sancak B, et al. PERFORMANCE EVALUATION OF SIX DIFFERENT SARS-CoV-2 ANTIBODY IMMUNOASSAYS: DISEASE SEVERITY AND SERUM SAMPLING TIME AFFECT THE SENSITIVITY. Jpn J Infect Dis 2022.
- Bolotin S, Tran V, Osman S, et al. SARS-CoV-2 Seroprevalence Survey Estimates Are Affected by Anti-Nucleocapsid Antibody Decline. J Infect Dis 2021; 223(8): 1334-8.
- Softeland JM, Gisslen M, Liljeqvist JA, et al. Longevity of anti-spike and anti-nucleocapsid antibodies after COVID-19 in solid organ transplant recipients compared to immunocompetent controls. Am J Transplant 2022; 22(4): 1245-52.
- Addetia AC, K. H. D.: Dingens, A.: Zhu, H.: Roychoudhury, P.: Huang, M. L.: Jerome, K. R.: Bloom, J. D.: Greninger, A. L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J Clin Microbiol 2020; 58(11).
- Ali AMA, K. M.: Fatah, M. H.: Tawfeeq, H. M.: Rostam, H. M. SARS-CoV-2 reinfection in patients negative for immunoglobulin G following recovery from COVID-19. New Microbes New Infect 2021; 43: 100926.
- Atti A, Insalata F, Carr EJ, et al. Antibody correlates of protection from SARS-CoV-2 reinfection prior to vaccination: A nested case-control within the SIREN study. (1532-2742 (Electronic)).
- Hanrath AT, Payne BAI, Duncan CJA. Prior SARS-CoV-2 infection is associated with protection against symptomatic reinfection. Journal of Infection 2021; 82(4): e29-e30.
- Hønge BL, Hindhede L, Kaspersen KA, et al. Long-term detection of SARS-CoV-2 antibodies after infection and risk of re-infection. Int J Infect Dis 2022; 116: 289-92.
- Lumley SFOD, D.: Stoesser, N. E.: Matthews, P. C.: Howarth, A.: Hatch, S. B.: Marsden, B. D.: Cox, S.: James, T.: Warren, F.: Peck, L. J.: Ritter, T. G.: de Toledo, Z.: Warren, L.: Axten, D.: Cornall, R. J.: Jones, E. Y.: Stuart, D. I.: Screaton, G.: Ebner, D.: Hoosdally, S.: Chand, M.: Crook, D. W.: O'Donnell, A. M.: Conlon, C. P.: Pouwels, K. B.: Walker, A. S.: Peto, T. E. A.: Hopkins, S.: Walker, T. M.: Jeffery, K.: Eyre, D. W. Antibody Status and Incidence of SARS-CoV-2 Infection in Health Care Workers. N Engl J Med 2021; 384(6): 533-40.
- Asderakis A, Khalid U, Koimtzis G, et al. An Analysis of Serological Response and Infection Outcomes Following Oxford-AstraZeneca (AZD1222) and Pfizer-BioNTech (mRNA BNT162b2) SARS-CoV-2 Vaccines in Kidney and Kidney-pancreas Transplants. Transplantation 2022; 106(7): 1421-9.
- Anand S, Montez-Rath ME, Han J, et al. SARS-CoV-2 Vaccine Antibody Response and Breakthrough Infection in Patients Receiving Dialysis. Annals of Internal Medicine 2022; 175(3): 371-8.
- Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. The Lancet Microbe 2022; 3(1): e52-e61.
- Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375(6576): 43-50.
- McGee C, Shi M, House J, et al. Longitudinal Serological Surveillance for COVID-19 Antibodies after Infection and Vaccination. Microbiology Spectrum 2022; 10(5).
- Murt A, Altiparmak MR, Yadigar S, et al. Antibody responses to the SARS-CoV-2 vaccines in hemodialysis patients: Is inactivated vaccine effective? Ther Apher Dial 2022; 26(4): 769-74.
- Ollila TA, Masel RH, Reagan JL, et al. Seroconversion and outcomes after initial and booster COVID-19 vaccination in adults with hematologic malignancies. Cancer 2022.
- Patnaik AP, Rout NK, Ahmed S, Dash KA, Praharaj AK, Patro ARK. Correlation of Breakthrough Infection During the Omicron Wave With Seropositivity of Vaccinated Patients Undergoing Hemodialysis. Cureus 2022; 14(9): e29296.
- Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med 2021; 385(16): 1474-84.
- Aldridge RW, Yavlinsky A, Nguyen V, et al. SARS-CoV-2 antibodies and breakthrough infections in the Virus Watch cohort. Nat Commun 2022; 13(1): 4869.
- Senefeld JW, Franchini M, Mengoli C, et al. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw Open 2023; 6(1): e2250647.
- Bloch EM, Focosi D, Shoham S, et al. Guidance on the Use of Convalescent Plasma to Treat Immunocompromised Patients With Coronavirus Disease 2019. Clin Infect Dis 2023; 76(11): 2018-24.
- Estcourt LJ, Cohn CS, Pagano MB, et al. Clinical Practice Guidelines From the Association for the Advancement of Blood and Biotherapies (AABB): COVID-19 Convalescent Plasma. Ann Intern Med 2022; 175(9): 1310-21.
- Cesaro S, Ljungman P, Mikulska M, et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 2022; 36(6): 1467-80.
- Centers for Disease Control and Prevention. About MIS. Available at: https://www.cdc.gov/mis/about.html. Accessed 10 May 2023.
- Kristiansen PA, Page M, Bernasconi V, et al. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021; 397(10282): 1347-8.
- U.S. Food and Drug Administration. cPass SARS-CoV-2 Neutralization Antibody Detection Kit. Available at: https://www.fda.gov/media/143583/download. . Accessed 28 April 2023.
- Long Q-X, Tang X-J, Shi Q-L, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nature Medicine 2020.
- World Health Organization. WHO/BS.2020.2403 Establishment of the WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody. Available at: https://www.who.int/publications/m/item/WHO-BS-2020.2403. Accessed 27 August 2023.
Supplementary Information
Supplement A
- Figure s1. PRISMA Flow Diagram
- Table s1. PICO questions Identified by the Panel through the two guideline versions
- Table s2. Search Strategies
- Table s3. QUADAS-2 Risk of Bias Assessment (for test accuracy studies)
- Table s4. ROBINS-I Risk of Bias Assessment (for non-test accuracy comparative studies)
- Table s6. Narrative summaries of studies informing question around performing serology testing in people with negative NAAT
- Table s7. Narrative summaries of studies informing question around performing serology testing in people with suspected MISC
- Table s8. Narrative summaries of studies informing question around performing serology testing in people with history of COVID-19 infection
- Table s9. Narrative summaries of studies informing question around performing serology testing in people with history of COVID-19 vaccination
Supplement B
- Figure s2a. Forest plot for the sensitivity of week 1 IgM using NAAT as reference standard
- Figure s2b. Forest plot for the specificity of week 1 IgM using NAAT as reference standard
- Figure s3a. Forest plot for the sensitivity of week 2 IgM using NAAT as reference standard
- Figure s3b. Forest plot for the specificity of week 2 IgM using NAAT as reference standard
- Figure s4a. Forest plot for the sensitivity of week 1 IgG using NAAT as reference standard
- Figure s4b. Forest plot for the specificity of week 1 IgG using NAAT as reference standard
- Figure s5a. Forest plot for the sensitivity of week 2 IgG using NAAT as reference standard
- Figure s5b. Forest plot for the specificity of week 2 IgG using NAAT as reference standard
- Figure s6a. Forest plot for the sensitivity of week 1 IgM or IgG combination tests using NAAT as reference standard
- Figure s6b. Forest plot for the specificity of week 1 IgM or IgG combination tests using NAAT as reference standard
- Figure s7a. Forest plot for the sensitivity of week 2 IgM or IgG combination tests using NAAT as reference standard
- Figure s7b. Forest plot for the specificity of week 2 IgM or IgG combination tests using NAAT as reference standard
Supplement C
- Figure s8a. Forest plot for the sensitivity of week 3 IgM using NAAT as reference standard
- Figure s8b. Forest plot for the specificity of week 3 IgM using NAAT as reference standard
- Figure s9a. Forest plot for the sensitivity of week 4 IgM using NAAT as reference standard
- Figure s9b. Forest plot for the specificity of week 4 IgM using NAAT as reference standard
- Figure s10a. Forest plot for the sensitivity of week 5 IgM using NAAT as reference standard
- Figure s10b. Forest plot for the specificity of week 5 IgM using NAAT as reference standard
- Figure s11a. Forest plot for the sensitivity of week 6 IgM using NAAT as reference standard
- Figure s11b. Forest plot for the specificity of week 6 IgM using NAAT as reference standard
- Figure s12a. Forest plot for the sensitivity of week 7 IgM using NAAT as reference standard
- Figure s12b. Forest plot for the specificity of week 7 IgM using NAAT as reference standard
- Figure s13a. Forest plot for the sensitivity of week 8 IgM using NAAT as reference standard
- Figure s13b. Forest plot for the specificity of week 8 IgM using NAAT as reference standard
- Figure s14a. Forest plot for the sensitivity of week 3 IgG using NAAT as reference standard
- Figure s14b. Forest plot for the specificity of week 3 IgG using NAAT as reference standard
- Figure s15a. Forest plot for the sensitivity of week 4 IgG using NAAT as reference standard
- Figure s15b. Forest plot for the specificity of week 4 IgG using NAAT as reference standard
- Figure s16a. Forest plot for the sensitivity of week 5 IgG using NAAT as reference standard
- Figure s16b. Forest plot for the specificity of week 5 IgG using NAAT as reference standard
- Figure s17a. Forest plot for the sensitivity of week 6 IgG using NAAT as reference standard
- Figure s17b. Forest plot for the specificity of week 6 IgG using NAAT as reference standard
- Figure s18a. Forest plot for the sensitivity of week 7 IgG using NAAT as reference standard
- Figure s18b. Forest plot for the specificity of week 7 IgG using NAAT as reference standard
- Figure s19a. Forest plot for the sensitivity of week 8 IgG using NAAT as reference standard
- Figure s19b. Forest plot for the specificity of week 8 IgG using NAAT as reference standard
- Figure s20a. Forest plot for the sensitivity of week 3 IgM or IgG combination tests using NAAT as reference standard
- Figure s20b. Forest plot for the specificity of week 3 IgM or IgG combination tests using NAAT as reference standard
- Figure s21a. Forest plot for the sensitivity of week 4 IgM or IgG combination tests using NAAT as reference standard
- Figure s21b. Forest plot for the specificity of week 4 IgM or IgG combination tests using NAAT as reference standard
- Figure s22a. Forest plot for the sensitivity of week 5 IgM or IgG combination tests using NAAT as reference standard
- Figure s22b. Forest plot for the specificity of week 5 IgM or IgG combination tests using NAAT as reference standard
- Figure s23a. Forest plot for the sensitivity of week 6 IgM or IgG combination tests using NAAT as reference standard
- Figure s23b. Forest plot for the specificity of week 6 IgM or IgG combination tests using NAAT as reference standard
- Figure s24a. Forest plot for the sensitivity of week 3 Total Antibodies using NAAT as reference standard
- Figure s24b. Forest plot for the specificity of week 3 Total Antibodies using NAAT as reference standard
- Figure s25a. Forest plot for the sensitivity of week 4 Total Antibodies using NAAT as reference standard
- Figure s25b. Forest plot for the specificity of week 4 Total Antibodies using NAAT as reference standard
- Figure s26a. Forest plot for the sensitivity of week 5 Total Antibodies using NAAT as reference standard
- Figure s26b. Forest plot for the specificity of week 5 Total Antibodies using NAAT as reference standard
- Figure s27a. Forest plot for the sensitivity of week 6 Total Antibodies using NAAT as reference standard
- Figure s27b. Forest plot for the specificity of week 6 Total Antibodies using NAAT as reference standard
- Figure s28a. Forest plot for the sensitivity of week 7 Total Antibodies using NAAT as reference standard
- Figure s28b. Forest plot for the specificity of week 7 Total Antibodies using NAAT as reference standard
Supplement D
- Figure s29a. Forest plot for the sensitivity of IgG assays targeting spike using NAAT as reference standard
- Figure s29b. Forest plot for the specificity of IgG assays targeting spike using NAAT as reference standard
- Figure s30a. Forest plot for the sensitivity of IgG assays targeting Nucleocapsid using NAAT as reference standard
- Figure s30b. Forest plot for the specificity of IgG assays targeting Nucleocapsid using NAAT as reference standard
Publication Disclaimer
© IDSA <2024>. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, transmitted, used for text and data mining, or used for training artificial intelligence, in any form or by any means, without the prior permission in writing, or as expressly permitted by law, by license or under terms agreed with the appropriate reprographics rights organization. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through the Oxford University Press RightsLink service via the Permissions link for this paper in Clinical Infectious Diseases. For further information please contact journals.permissions@oup.com.