COVID-19 Guideline: Treatment and Management
Published by IDSA,
COVID-19 Guideline, Part 2: Infection Prevention
COVID-19 Guideline, Part 3: Molecular Testing
COVID-19 Guideline, Part 4: Serologic Testing
COVID-19 Guideline, Part 5: Antigen Testing
Management of Drug Interactions With Nirmatrelvir/Ritonavir (Paxlovid®): Resource for Clinicians
Adarsh Bhimraj,* Rebecca L. Morgan,** Amy Hirsch Shumaker, Lindsey Baden, Vincent Chi-Chung Cheng, Kathryn M. Edwards, Jason C. Gallagher, Rajesh T. Gandhi, William J. Muller, Mari M. Nakamura, John C. O’Horo, Robert W. Shafer, Shmuel Shoham, M. Hassan Murad,** Reem A. Mustafa,** Shahnaz Sultan,** Yngve Falck-Ytter**
*Corresponding Author **Methodologist
June 26, 2023
Version 11.0.0 has been released and includes the following:
- Convalescent Plasma: A new recommendation was developed against the routine use of convalescent plasma among immunocompromised patients hospitalized with COVID-19. Additionally, this section includes updated remarks for the existing recommendation on the use of convalescent plasma for ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease who have no other treatment options.
- Anakinra: This section has been added and includes a new recommendation against the routine use of anakinra among hospitalized patients with severe COVID-19.
- Nirmatrelvir/Ritonavir: This section includes updated remarks for the existing recommendation on the use of nirmatrelvir/ritonavir for ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease.
This update has been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.

Strengths of recommendation
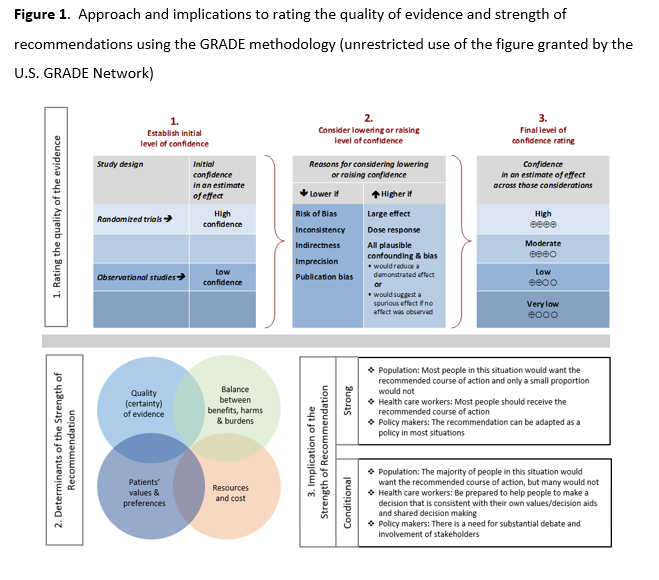
Recommend (strong recommendation): Guideline panel is confident that the desirable effects of an intervention outweigh the undesirable effects. Most or all individuals will be best served by the recommended course of action.
Suggest (weak or conditional recommendation): Guideline panel after discussion concludes that the desirable effects probably outweigh undesirable effects, but appreciable uncertainty exists. Not all individuals will be best served by the recommended course of action and the caregiver needs to consider more carefully than usual the individual patient’s circumstances, preferences, and values.
Certainty of evidence
⨁⨁⨁⨁ high
⨁⨁⨁◯ moderate
⨁⨁◯◯ low
⨁◯◯◯ very low
See Figure 1 below.
Background: There are many pharmacologic therapies that are being used or considered for treatment of coronavirus disease 2019 (COVID-19), with rapidly changing efficacy and safety evidence from trials.
Objective: Develop evidence-based, rapid, living guidelines intended to support patients, clinicians, and other healthcare professionals in their decisions about treatment and management of patients with COVID-19.
Methods: In March 2020, the Infectious Diseases Society of America (IDSA) formed a multidisciplinary guideline panel of infectious disease clinicians, pharmacists, and methodologists with varied areas of expertise to regularly review the evidence and make recommendations about the treatment and management of persons with COVID-19. The process used a living guideline approach and followed a rapid recommendation development checklist. The panel prioritized questions and outcomes. A systematic review of the peer-reviewed and grey literature was conducted at regular intervals. The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to assess the certainty of evidence and make recommendations.
Results: Based on the most recent search conducted on May 31, 2022, the IDSA guideline panel has made 29 recommendations for the treatment and management of the following groups/populations: pre- and post-exposure prophylaxis, ambulatory with mild-to-moderate disease, hospitalized with mild-to-moderate, severe but not critical, and critical disease. As these are living guidelines, the most recent recommendations can be found online at: https://idsociety.org/COVID19guidelines.
Conclusions: At the inception of its work, the panel has expressed the overarching goal that patients be recruited into ongoing trials. Since then, many trials were done which provided much needed evidence for COVID-19 therapies. There still remain many unanswered questions as the pandemic evolved which we hope future trials can answer.
Keywords: coronavirus, SARS-CoV-2, COVID, COVID-19, pneumonia
Executive Summary
Coronavirus disease 2019 (COVID-19) is a pandemic with a rapidly increasing incidence of infections and deaths. Many pharmacologic therapies are being used or considered for treatment. Given the rapidity of emerging literature, the Infectious Diseases Society of America (IDSA) identified the need to develop living, frequently updated evidence-based guidelines to support patients, clinicians and other health-care professionals in their decisions about treatment and management of patients with COVID-19. Please refer to the IDSA website for the latest version of the guidelines: https://idsociety.org/COVID19guidelines.
A detailed description of background, methods, evidence summary and rationale that support each recommendation, and research needs can be found online in the full text. In brief, per Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology, recommendations are labeled as “strong” or “conditional”. The word “recommend” indicates strong recommendations and “suggest” indicates conditional recommendations. In situations where promising interventions were judged to have insufficient evidence of benefit to support their use and with potential appreciable harms or costs, the expert panel recommended their use in the context of a clinical trial. These recommendations acknowledge the current “knowledge gap” and aim at avoiding premature favorable recommendations for potentially ineffective or harmful interventions.
Background
The first cases of COVID-19 were reported from Wuhan, China in early December 2019 [1], now known to be caused by a novel beta-coronavirus, named as Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Within a span of months, COVID-19 has become pandemic due to its transmissibility, spreading across continents with the number of cases and deaths rising daily [2]. The emergence of new variants as the pandemic evolved has added more challenges to the prevention and treatment of COVID-19. Although most infected individuals exhibit a mild illness (80%+), 14% have serious and 5% have critical illness. Approximately 10% will require hospital admission due to COVID-19 pneumonia, of which approximately 10% will require intensive care, including invasive ventilation due to acute respiratory distress syndrome (ARDS) [3]. While mortality appears to be more common in older individuals and those with comorbidities, such as chronic lung disease, cardiovascular disease, hypertension and diabetes, young people with no comorbidities also appear to be at risk for critical illness including multi-organ failure and death.
There has been an expanding number of studies rapidly published online and in academic journals; however, some of these may be of limited quality and are pre-published without sufficient peer-review. Critical appraisal of the existing studies is needed to determine if the existing evidence is sufficient to support currently proposed management strategies.
Given the rapid global spread of SARS-CoV-2 and the difficulty for the overburdened front-line providers and policymakers to stay up to date on emerging literature, IDSA has recognized the necessity of developing a rapid guideline for the treatment of COVID-19. The guideline panel is using a methodologically rigorous process for evaluating the best available evidence and providing treatment recommendations. These guidelines will be frequently updated as substantive literature becomes available and are accessible on an easy to navigate web and device interface at http://www.idsociety.org/covid19guidelines.
There continue to be several ongoing trials evaluating therapeutic agents for the treatment of COVID-19. As data becomes available from these trials and if there is a preponderance of evidence to suggest the use of a therapeutic agent even in the context of clinical trials is no longer warranted it will be removed from future updates of the guideline (and the removal will be noted in the updated guidelines). If there is emerging evidence on the efficacy or safety of a therapeutic agent not mentioned in the current version of the guideline it will be included in future updates of the guideline.
These recommendations are intended to inform patients, clinicians, and other health professionals by providing the latest available evidence.
In this section, we discuss how to approach a patient suspected to have COVID-19 and how to apply the IDSA COVID-19 treatment guidelines to specific clinical syndromes. The detailed evidence appraisals and recommendations for each therapeutic agent can be found in the individual sections. The certainty of supporting evidence is low to moderate for most recommendations; therefore, the guideline panel made conditional suggestions rather than strong recommendations for or against most of the agents. Though substantial progress was made with COVID-19 therapies in such a short period, there still remain many unanswered questions in the management of COVID-19. Therefore, the approach outlined here and in the guidelines are based on some assumptions and extrapolations. Despite limited evidence, to give actionable and timely guidance to frontline clinicians, we provide recommendations for use of combinations of agents, recommend some agents over others or extrapolate to sub populations not evaluated in trials.
Some of the critical unanswered questions in COVID-19 treatment trials are:
- Which sub-populations or specific clinical types of patients with COVID-19 benefit most from specific therapeutic agents?
- What is the efficacy and safety of COVID-19 therapies in populations that are immune from prior SARS-CoV-2 infections and vaccination?
- What is the efficacy and safety of treatments in infections with specific SARS-CoV-2 variants and sub-variants?
- How do therapeutic agents perform when compared to each other to allow a tiered approach to treating patients with COVID-19?
- What is the comparative efficacy and safety of nirmatrelvir/ritonavir versus remdesivir, molnupiravir, and different anti-SARS-CoV-2 antibodies in mild-to-moderate disease?
- What is the efficacy and safety of IL-6 inhibitors when compared to JAK inhibitors in severe disease?
- What is the comparative efficacy and safety of combinations of different drugs in treating different severities and clinical phenotypes of COVID-19?
- Which biomarkers can be used as predictors of therapeutic response to specific agents?
We hope future studies and trials address these uncertainties so we can give a more definitive treatment approach to COVID-19.
General principles of COVID-19 pharmacotherapy
During the early phase of the infection, when viral load is high and the host’s adaptive immune system has not mounted an adequate response, treatments targeting viral replication are most likely to be effective. These include both the direct antiviral therapies nirmatrelvir/ritonavir, molnupiravir, and remdesivir; and the passive immunity therapies of anti-SARS-CoV-2 antibodies and donor convalescent plasma. Timely initiation of antiviral therapies is critical as they are more efficacious when given within 5 to 7 days of symptom onset. Most patients do not progress to severe or critical disease, but some with risk factors do. Later in the disease process, in patients with severe and especially critical disease, an excessive and aberrant inflammatory response is implicated to be the primary cause of immunopathological damage. At this stage anti-inflammatory therapies like corticosteroids, IL-6 inhibitors or JAK inhibitors have been shown to be beneficial.
Clinical evaluation
Clinical evaluation should consider patient and pathogen specific factors that can influence choice of COVID-19 treatments. The evaluation should at least include assessment of:
- Severity of COVID-19
- Date of onset of symptoms
- Risk factors for progression to severe disease or death (see further discussion below, under Pharmacologic treatment of mild-to-moderate COVID-19 with risk factors for progression)
- Degree of chronic and acute end-organ dysfunction (including, but not limited to, pulmonary, cardiovascular, renal, and hepatic)
- Age and pregnancy status
- Virus-specific factors that may influence the choice of pharmacotherapy (e.g., variant specific susceptibility to certain drugs)
- Risk factors for progression are changing as the epidemic evolves with new variants, vaccination, and previous infection rates.
Diagnostic classification of severity of COVID-19 helps target specific treatments to patient populations that have been demonstrated to benefit in COVID-19 treatment trials. The clinician should identify which of the severity categories in Table 1 the patient falls into.
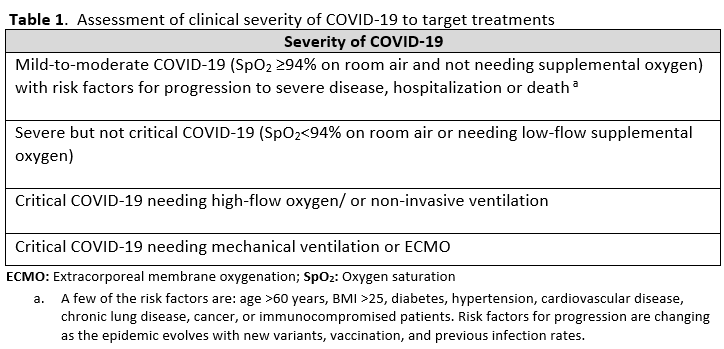
It is also important to identify factors that preclude the use of COVID-19 treatments or warrant their use with caution. Patients with low estimated GFR were not included in the trials for remdesivir and tocilizumab. Elevated aspartate transaminase (AST) and alanine transaminase (ALT) levels are a contraindication for IL-6 inhibitors and remdesivir. Patients who were neutropenic, had an active bacterial, fungal or parasitic infection, or were hypercoagulable were eliminated from some of the JAK inhibitor trials. It is also important to identify if the patients have other acute disease that either mimic COVID-19 or present concomitantly with COVID-19. Patients can have a positive SARS-CoV-2 by RT-PCR from a nasopharyngeal sample, and present with pulmonary disease caused by a bacterial pneumonia or pulmonary edema. Patients with COVID-19 can also have pulmonary embolism contributing to their symptoms and hypoxemia. It is important to avoid anchoring bias to the diagnosis of COVID-19 and be attentive to considering and evaluating other etiologies. Many of the COVID-19 therapies are not FDA-approved and have instead received FDA EUA, so it is necessary to follow the regulatory processes and protocols for these agents.
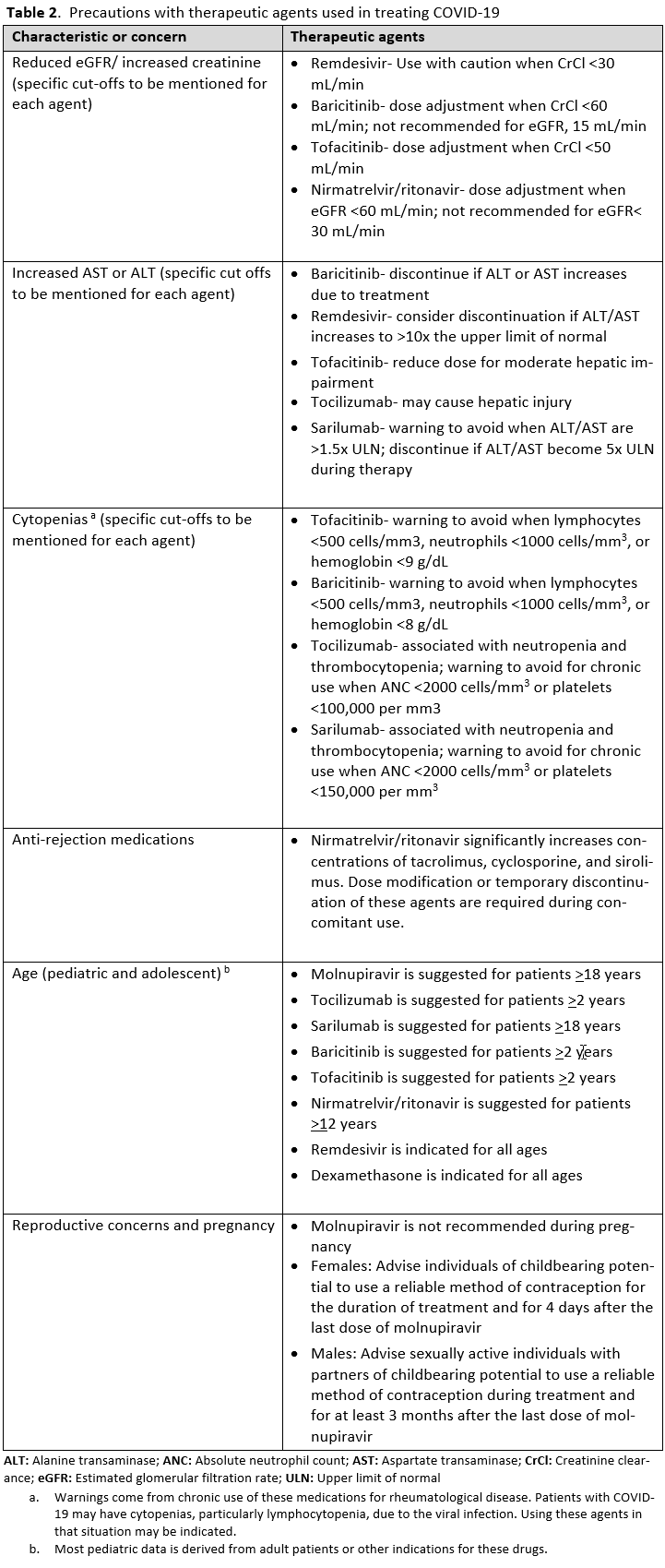
Pharmacologic treatment of mild-to-moderate COVID-19 with risk factors for progression
COVID-19 is considered mild when there are clinical features suggestive of upper respiratory tract involvement without features of lung or other end organ involvement. Moderate COVID-19 is pulmonary involvement with no hypoxia. Most patients improve with supportive care at this stage, but patients with risk factors can progress to more severe or critical disease or death; such individuals may benefit from pharmacotherapies. There are no validated clinical prediction rules or risk calculators, but the FDA EUA and CDC mention a few of these risk factors to consider for treatment with anti-SARS-CoV-2 antibodies [1]. More research is needed to identify prediction instruments and determinants that both increase or decrease the risk of severe disease and how potentially protective factors influence risk stratification. Most of these treatments are effective only when given early, within 5-7 days of symptom onset.
Patients who have these risk factors should be offered treatment with nirmatrelvir/ritonavir for 5 days (oral) or remdesivir for 3 days (intravenous). If these agents are not available or cannot be used then consider molnupiravir for 5 days (oral) or, if immunocompromised, high-titer convalescent plasma (intravenous) with activity against circulating variant. Convalescent plasma obtained from people who have recovered from COVID-19 due to Omicron and have been vaccinated is expected to be active against Omicron.
Parenteral anti-SARS-CoV-2 monoclonal antibodies can be used to treat if the circulating SARS CoV-2 variants in that region are susceptible to the specific agent, given trials have shown a reduction in the need for hospitalizations, ER visits or medically attended visit. At present (2/2/2023) a significant proportion of the circulating SARS CoV-2 variants in the US are not susceptible to most of the neutralizing antibodies. There are no neutralizing antibodies that are currently (2/2/2023) authorized or approved by US FDA.
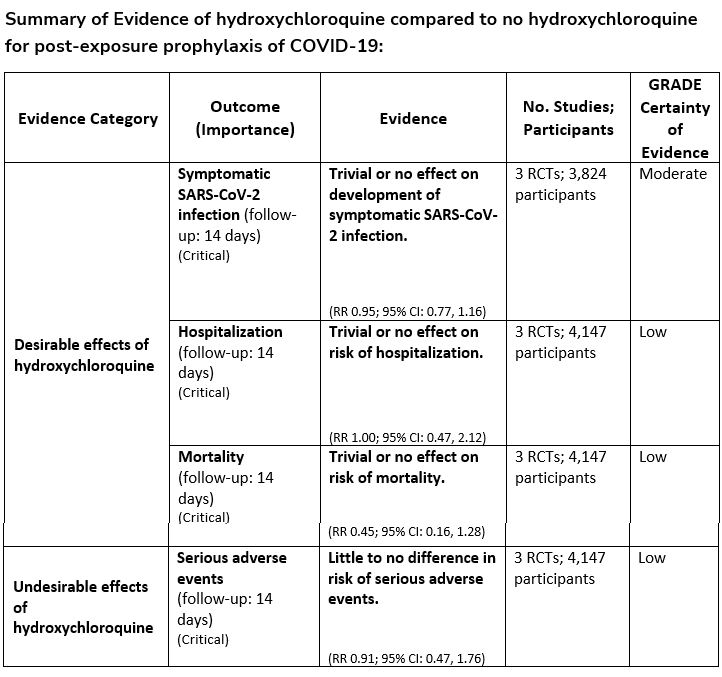
There are logistical issues related to administration of parenteral agents in ambulatory settings which may preclude their use. Oral antivirals like nirmatrelvir/ritonavir and molnupiravir have an advantage as they are easy to prescribe in outpatient settings, but there are significant limitations and unique considerations that need to be addressed by providers, which might be a barrier to their timely use. In the United States, many of the antiviral treatments do not have authorization for use in patients admitted to the hospital for mild-to-moderate COVID-19 but can be used if they are admitted for another reason and found to have mild-to-moderate COVID-19. We do not recommend using hydroxychloroquine, azithromycin, or lopinavir/ritonavir as trials have shown no evidence of benefit.
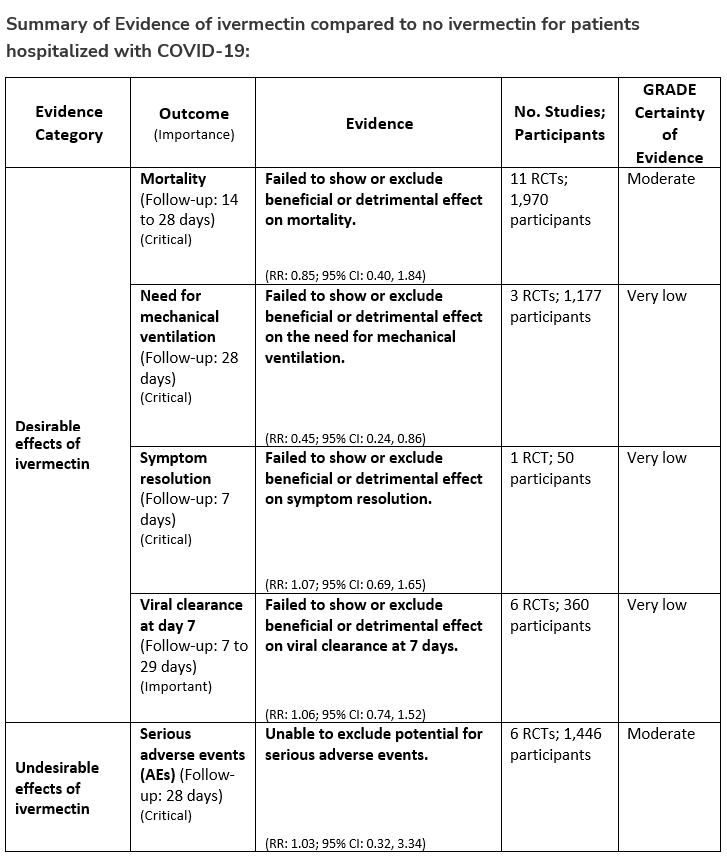
We recommend against the use of ivermectin outside of the context of a clinical trial given the low certainty of evidence for its benefit. We also do not recommend the use of systemic corticosteroids in mild-to-moderate COVID-19. Though the RECOVERY trial was completed in hospitalized patients and not ambulatory patients, it demonstrated a trend to increase mortality when used in patients with mild-to-moderate COVID-19 (relative risk 1.22; 95% CI 0.86, 1.75) [2].
Pharmacologic treatment of severe COVID-19
Patients with severe COVID-19 are those whose infection has pulmonary involvement resulting in hypoxia while breathing room air and/or needing treatment with low flow oxygen. Most existing criteria for trials consider either a SpO2 level less than 94% or 90% or tachypnea (respiratory rate >30 breaths per minute) as severe COVID-19. Clinical judgment of individual cases should supplement these criteria.
Corticosteroids, especially dexamethasone, has demonstrated a mortality benefit are recommended as the cornerstone of therapy in severe COVID-19. Remdesivir may be considered as it has shown to decrease time to recovery or discharge, though it has not been shown to improve mortality [3, 4].
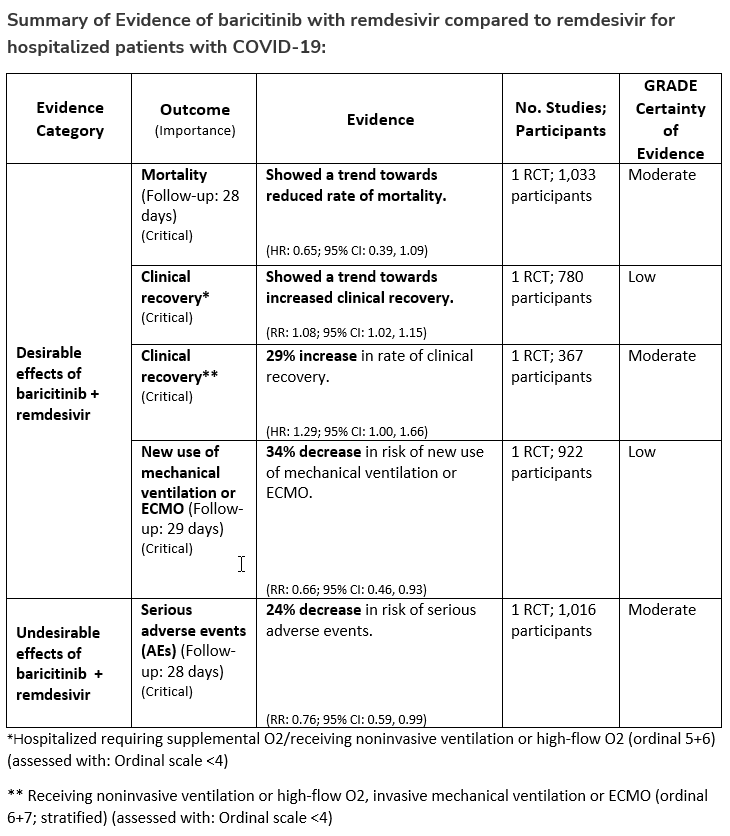
The IL-6 inhibitors tocilizumab and sarilumab [5, 6] and JAK inhibitors baricitinib and tofacitinib [7] have shown a benefit in severe, but non-critical COVID-19 when used with corticosteroids. The trials did not identify specific sub-populations of patients with severe COVID-19 already being treated with corticosteroids who would benefit most with additional treatment with IL-6 or JAK inhibitors. We recommend using either IL-6 inhibitors or JAK inhibitors (baricitinib preferred over tofacitinib) in those patients who have elevated inflammatory markers like CRP and progressive severe COVID-19. Since there is greater supportive data for tocilizumab and baricitinib we recommend them preferentially over sarilumab and tofacitinib, though the latter agents are suitable alternatives if the former are not available. We do not recommend using hydroxychloroquine, azithromycin, lopinavir/ritonavir, or convalescent plasma as trials have not shown a benefit in patients with severe disease. We also recommend against the use of ivermectin outside of the context of a clinical trial given the low certainty of evidence for its benefit.
Pharmacologic treatment of critically ill COVID-19 requiring non-invasive ventilation or oxygen by high-flow nasal cannula
Critically ill patients with COVID-19 need more ventilatory or oxygenation support either with high-flow oxygen or with noninvasive ventilation. High-flow oxygen therapy involves delivery of oxygen via special devices at rates greater than those possible via a simple nasal canula.
We strongly recommend systemic corticosteroids in critically ill patients with COVID-19 as they have shown a mortality benefit in this population (OR: 0.66; 95% CI: 0.54; 0.82) [8]. In critically ill patients, dexamethasone 6mg/day is preferred but doses up to 20 mg/day can be used if indicated for other reasons. Hydrocortisone 50 mg IV Q6 hours is an alternative that has also been studied. Methylprednisolone and prednisone have less supporting data but are reasonable pharmacologic alternatives at equipotent doses. In addition to corticosteroids, we recommend using either IL-6 inhibitors (tocilizumab preferred over sarilumab) or JAK inhibitors (baricitinib preferred over tofacitinib) in patients who have elevated inflammatory markers (e.g., CRP), which most critically ill COVID-19 patients have. The trials done so far have not identified specific sub-populations of critically ill patients already being treated with corticosteroids who would benefit with additional treatment with IL-6 or JAK inhibitors. We do not recommend remdesivir since it has not shown a benefit in this sub-population [3].
Pharmacologic treatment of critically ill COVID-19, needing invasive mechanical ventilation or ECMO
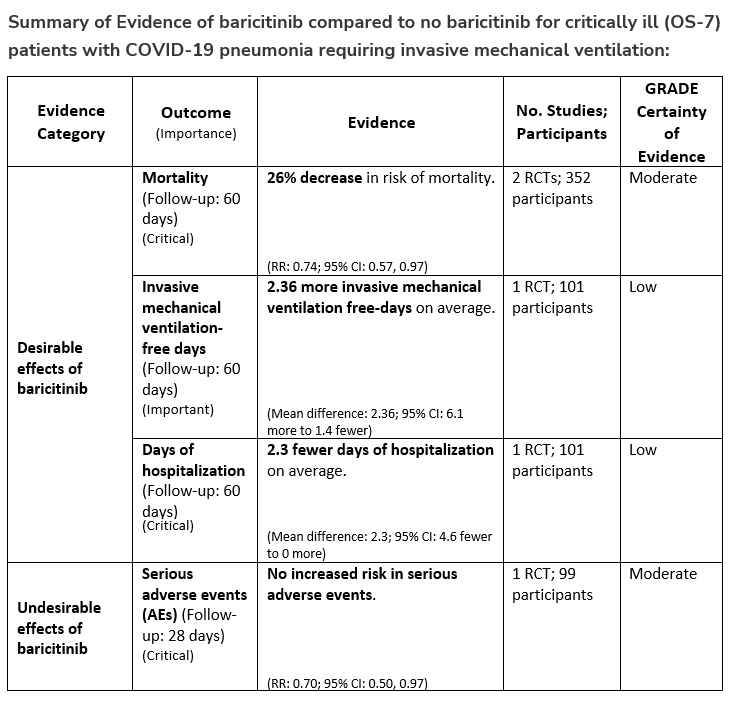
Patients who are critically ill with COVID-19 pulmonary disease and dysfunction needing significant ventilatory support with invasive mechanical ventilation or ECMO have the highest risk of mortality. Pharmacologically, we recommend treating them similarly to those on non-invasive ventilation or high-flow nasal cannula. Corticosteroids are strongly recommended in this category of critically ill patients as trials have demonstrated a mortality benefit [8]. In addition to steroids, the panel recommends using either IL-6 inhibitors (tocilizumab is preferred over sarilumab) in critically ill patients who have elevated inflammatory markers like CRP. In situations where IL-6 inhibitors are not available, baricitinib can be used in mechanically ventilated patients as a small trial showed a mortality benefit in this population [9]. Most other COVID-19 therapies studied in other severities have either not demonstrated benefit or not been studied in this population.
References
1. Centers for Disease Control and Prevention. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html. Accessed 20 June 2022.
2. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021; 384(8): 693-704.
3. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020; 383(19): 1813-26.
4. WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 2021; 384(6): 497-511.
5. Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet 2021; 397(10285): 1637-45.
6. WHO Rapid Evidence Appraisal for COVID-19 Therapies Working Group, Shankar-Hari M, Vale CL, et al. Association Between Administration of IL-6 Antagonists and Mortality Among Patients Hospitalized for COVID-19: A Meta-analysis. JAMA 2021; 326(6): 499-518.
7. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021; 9(12): 1407-18.
8. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020; 324(13): 1330-41.
9. Ely EW, Ramanan AV, Kartman CE, et al. Baricitinib plus Standard of Care for Hospitalised Adults with COVID-19 on Invasive Mechanical Ventilation or Extracorporeal Membrane Oxygenation: Results of a Randomised, Placebo-Controlled Trial. medRxiv 2021: Available at: https://doi.org/10.1101/2021.10.11.21263897 [Preprint 12 October 2021].
Patients with COVID-19 often present with viral pneumonia with accompanying febrile illness and respiratory symptoms. Differential diagnoses may include bacterial pneumonia, for which antibiotics are prescribed. Concerns also exist for bacterial superinfections in hospitalized patients during the course of illness. Studies reported to date mainly describe antibiotic use during the early phase of the COVID-19 pandemic and consistently report high percentages of antibiotic use worldwide (58-95%) [1-8]. One registry of 150 Spanish hospitals found that over 75% of patients received antibiotics, but diagnosis in the early months of the pandemic was a predictor of inappropriate antibiotic use. Antibiotic use was associated with adverse drug reactions [9].
Data reporting co-infection in patients presenting with COVID-19 for care has mostly focused on patients receiving care in hospitals. As more studies have become available, they can be grouped into those describing co-infection at the diagnosis of COVID-19, those describing the treatment of superinfections during the course of COVID-19 infection, those that report both, and those that do not distinguish between these types of infections. The latter are not discussed here.
Despite the majority of patients with COVID-19 being treated with antibiotics on admission early in the pandemic, existing studies have found bacterial co-infections to be uncommon. Vaughn and colleagues evaluated a random cohort of patients with COVID-19 across 38 hospitals in Michigan. Of the 1705 patients included, only 3.5% had a bacterial co-infection, though 59.5% received antibacterial drugs [6]. A cohort of 1016 patients with COVID-19 across five Maryland hospitals found bacterial co-infection in only 1.2% [10]. A meta-analysis including 3338 patients in 24 studies reported bacterial co-infection in 3.5% [11]. Smaller studies had congruent reports, ranging from 3.1 to 4% [12-14]. A study of 64,961 COVID-19 patients in the Premier Healthcare Database is an outlier, reporting bacterial co-infections in 18.5% of infections between April and June 2020, but this relied on ICD-10 codes and not microbiological diagnoses. Urinary tract infections were most reported [15].
Studies describing superinfections that developed in patients with COVID-19 are more heterogeneous. Studies that describe the incidence of superinfection in entire hospitalized cohorts of COVID-19 report incidences of superinfection of 4.2 to 21% [11, 14, 16]. Small studies of patients requiring mechanical ventilation and with COVID-19-associated ARDS reported superinfections in 44.4% and 27.7% of patients, respectively [17, 18].
The apparent discordance between bacterial and fungal co-infection in patients with COVID-19 at presentation and the use of antibacterial therapy has potential negative effects, namely in antimicrobial resistance. Several studies have attempted to differentiate patients with and without concomitant bacterial infections using laboratory data. The use of procalcitonin in a group of hospitals was not effective as tool to encourage antibiotic discontinuation compared to clinical judgment [19]. Mason and colleagues compared hospitalized cohorts of 619 patients with COVID-19 and 106 with community-acquired bacterial pneumonia (CABP) to determine if inflammatory markers could be used to rule out bacterial co-infection [20]. They found marked differences in white blood cell counts between groups (6.78 COVID-19 vs. 12.48 CABP), and that CRP declined in 48-72 hours with antibiotic therapy in the CABP cohort but not the COVID-19 group, suggesting that these can be used to guide antibiotic discontinuation when initiated empirically in COVID-19 patients. Initiating and continuing empiric antibiotics at the time of admission may lead to superinfections that are antibiotic resistant; one study found antibiotic use in the first two days of admission for COVID-19 to be a risk factor for superinfection [16]. Immunomodulatory therapies are recommended for many patients with severe and critical illness from COVID-19, including corticosteroids, IL-6 antagonists, JAK inhibitors, and others [21]. Most of the prospective studies that support these recommendations have not reported higher rates of infection in patients receiving immunomodulators, but follow-up is limited in most cases and late infections may be missed.
References
1. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395(10223): 507-13.
2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395(10229): 1054-62.
3. Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med 2020; 382(18): 1708-20.
4. Rojo JMC, Santos JMA, Núñez-Cortés JM, et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: results from the SEMI-COVID-19 Network. medRxiv 2020.
5. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 Patients with COVID-19 in New York: retrospective case series. medRxiv 2020.
6. Vaughn VM, Gandhi TN, Petty LA, et al. Empiric Antibacterial Therapy and Community-onset Bacterial Coinfection in Patients Hospitalized With Coronavirus Disease 2019 (COVID-19): A Multi-hospital Cohort Study. Clin Infect Dis 2021; 72(10): e533-e41.
7. Mason CY, Kanitkar T, Richardson CJ, et al. Exclusion of bacterial co-infection in COVID-19 using baseline inflammatory markers and their response to antibiotics. J Antimicrob Chemother 2021; 76(5): 1323-31.
8. Townsend L, Hughes G, Kerr C, et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist 2020; 2(3): dlaa071.
9. Calderon-Parra J, Muino-Miguez A, Bendala-Estrada AD, et al. Inappropriate antibiotic use in the COVID-19 era: Factors associated with inappropriate prescribing and secondary complications. Analysis of the registry SEMI-COVID. PLoS One 2021; 16(5): e0251340.
10. Karaba SM, Jones G, Helsel T, et al. Prevalence of Co-infection at the Time of Hospital Admission in COVID-19 Patients, A Multicenter Study. Open Forum Infect Dis 2021; 8(1): ofaa578.
11. Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26(12): 1622-9.
12. Adler H, Ball R, Fisher M, Mortimer K, Vardhan MS. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe 2020; 1(2): e62.
13. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin Microbiol Infect 2020; 26(10): 1395-9.
14. Garcia-Vidal C, Sanjuan G, Moreno-Garcia E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect 2021; 27(1): 83-8.
15. Baghdadi JD, Coffey KC, Adediran T, et al. Antibiotic Use and Bacterial Infection among Inpatients in the First Wave of COVID-19: a Retrospective Cohort Study of 64,691 Patients. Antimicrob Agents Chemother 2021; 65(11): e0134121.
16. Smith L, Karaba SM, Amoah J, et al. Hospital-acquired infections among adult patients admitted for coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol 2021: 1-4.
17. Kreitmann L, Monard C, Dauwalder O, Simon M, Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med 2020; 46(9): 1787-9.
18. Dudoignon E, Camelena F, Deniau B, et al. Bacterial Pneumonia in COVID-19 Critically Ill Patients: A Case Series. Clin Infect Dis 2021; 72(5): 905-6.
19. Fabre V, Karaba S, Amoah J, et al. The role of procalcitonin results in antibiotic decision-making in coronavirus disease 2019 (COVID-19). Infect Control Hosp Epidemiol 2021: 1-6.
20. Matsubara JA, Phillips DP. Intracortical connections and their physiological correlates in the primary auditory cortex (AI) of the cat. J Comp Neurol 1988; 268(1): 38-48.
21. Clancy CJ, Nguyen MH. COVID-19, superinfections and antimicrobial development: What can we expect? Clin Infect Dis 2020.
Acute SARS-CoV-2 Infection in Children
Clinical presentation
Case [1, 2] and hospitalization rates [3] from SARS-CoV-2 infection in children are lower than in adults, and asymptomatic infection is more common [4, 5]. However, infection can lead to significant illness and even death in children [6-8]. Clinical presentations of infection can be non-specific, and may more frequently include fever alone and/or gastrointestinal symptoms [9] than in adults. Children are also capable of transmitting disease to others [10].
Factors which lead to severe illness in children with SARS-CoV-2 infection are less well-defined than in adults. Comorbidities including medically complex conditions (including certain genetic disorders, neurologic diseases, and cancer) [11], type 1 diabetes, complex congenital heart disease, and obesity have all been associated with a higher risk of hospitalization and ICU admission in children [8, 12-14].
Management
Remdesivir
The studies involving the use of remdesivir in hospitalized patients with COVID-19 (recommendations 15-17) [15-19] have generally focused on individuals over age 18 years. Two trials included children over 12 years [16, 18], but did not separately report the number or outcomes (including adverse events) of participants under 18 years. Nevertheless, remdesivir is commonly used and recommended by expert panels [20] of pediatric ID specialists in hospitalized children with SARS-CoV-2 infection, and reports suggest low adverse event rates [21, 22]. An ongoing phase II/III open label study in children (the “CARAVAN” trial) [23] has not yet reported their results in the peer-reviewed literature [24]. Recent studies of outpatient remdesivir treatment in individuals at high risk for progression support its use in pediatric patients down to 3.5 kg of body weight.
Corticosteroids
Dexamethasone and other corticosteroids are recommended in certain hospitalized patients with COVID-19 (recommendations 7-9). The studies informing these recommendations [25, 26] either did not include children or did not separately report the number or outcomes (including adverse events) of participants under 18 [26] years. Corticosteroid use is nevertheless common in hospitalized children with COVID-19 [13], and there is reason to believe that the risk benefit ratio would be similar in children and adults.
IL-6 blockade
Tocilizumab or sarilumab is suggested for use in treatment of COVID-19 in certain situations (recommendations 11-12). Of the studies informing the recommendations for tocilizumab [27-34], only two [29, 34] did not specifically exclude children under 18 years from enrolling. The RECOVERY trial included children, but results from those in the tocilizumab arm of the trial have not yet been reported. Hermine et al. did not specifically exclude children, but results in children were not separately reported either.
Three of the four studies used to inform the recommendations for sarilumab excluded children from participation [27, 35, 36]. The pre-print network meta-analysis of 18 RCTs of IL-6 inhibitors included some studies that enrolled children, but results in children were not separately reported.
There are several publications reporting on cohorts of children with COVID-19 who received treatment with tocilizumab [22, 37-39]. Although there have been no clear contraindications to using IL-6 inhibitors in children based on these reports more studies in children are needed to determine whether the criteria for their pediatric use would be similar to those in adults.
JAK inhibitors
Baricitinib is suggested for use in treating certain hospitalized patients with COVID-19 (recommendations 21-22). However, the studies which inform these recommendations did not include children [40-43]. Although the EUA for use of baricitinib in treatment of COVID-19 extends to children over 2 years of age [44], baricitinib does not have an FDA indication for treatment of other conditions in children, and there are only limited published pediatric pharmacokinetic data [45]. A pediatric safety and pharmacokinetic study on baricitinib use in children with COVID-19 is now recruiting [46].
Tofacitinib is also suggested for use in treating certain hospitalized patients with COVID-19 (recommendation 23). As with baricitinib, the trial informing this recommendation did not include children [47]. Tofacitinib is used in children over age 2 and over 10 kg for treatment of polyarticular juvenile idiopathic arthritis when they have had an inadequate response or intolerance to one or more tumor necrosis factor inhibitors [48]. There are no currently open trials studying tofacitinib for treatment of COVID-19 in children.
Oral antivirals
Two new antiviral agents have been issued an EUA and include: nirmatrelvir/ritonavir and molnupiravir. Nirmatrelvir/ritonavir is not authorized in children younger than 12 years of age and weighing less than 40 kg [49]. However, there have been no safety or effectiveness studies in pediatric patients. Molnupiravir is not recommended for use in children due to animal studies that suggest effects on bone and cartilage growth.
Monoclonal antibodies
At earlier stages in the pandemic, neutralizing monoclonal antibodies directed against the spike protein of SARS-CoV-2 have been used for pre- and post-exposure prophylaxis and treatment of individuals exposed to or infected with SARS-CoV-2 who are at high risk of progression to severe disease, but emergence of variants with in vitro reductions in susceptibility to these antibodies has left no available products in the United States. As noted previously, use of these products may be considered in areas of the world where a significant proportion of circulating variants retain susceptibility, taking into account the predicted relative benefits of the anti-SARS CoV-2 neutralizing antibody product compared with alternative antiviral therapies. In children, clinicians should also consider limitations in the age ranges and minimum body weight in which these products have been studied and should note that risk factors for progression to severe illness in children are less well-defined than in adults. Although risk-benefit ratios for the use of SARS-CoV-2 monoclonal antibodies are likely similar between children and adults, pediatric-specific data are limited or lacking for all neutralizing monoclonal antibody products.
Treatments not recommended for use
As noted in other sections of this document, several interventions have been tested in adult populations and not found to have clinical benefit. This has led to recommendations against the routine use of hydroxychloroquine, lopinavir/ritonavir, inpatient convalescent plasma, and famotidine. Although the studies informing these recommendations largely excluded children with acute infection, the experience in adult patients suggests that these drugs would not be expected to have benefit in treatment of children with similar disease characteristics.
Multisystem Inflammatory Syndrome in Children
Clinical presentation
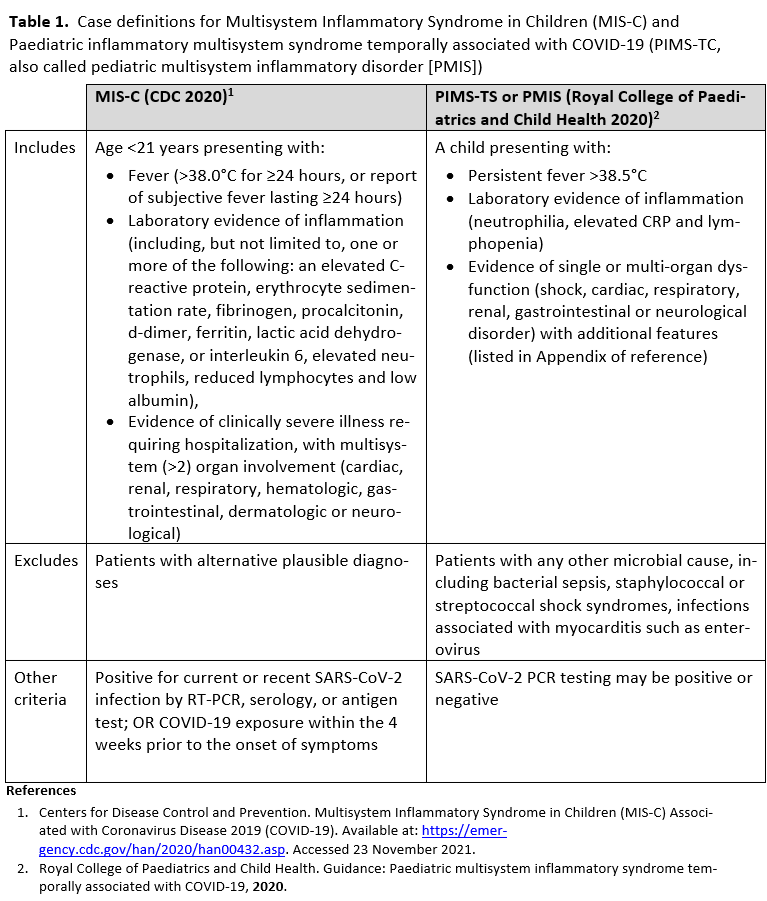
Multisystem inflammatory syndrome in children (MIS-C), also called Pediatric Inflammatory Multisystem Syndrome temporally associated with COVID-19 (PIMS-TS), is a rare acute inflammatory syndrome reported in children several weeks following acute SARS-CoV-2 infection. Case definitions for this syndrome were derived after reports of critically ill children presenting with fever, rash, conjunctivitis, abdominal complaints, shock, and significant cardiac dysfunction in the setting of recent SARS-CoV-2 infection [50-62] (Table 1). Incidence of MIS-C is higher in Black, Hispanic or Latinx, and Asian or Pacific Islander children than in Caucasian children and most common among children between 6 and 10 years of age [63, 64]. Epidemiologic data showing clusters of MIS-C cases following peaks of positive SARS-CoV-2 test rates by 2-5 weeks [65] support that the syndrome results from a delayed immunologic response to the infection.
Management
Once the diagnosis of MIS-C has been made, immunomodulatory medications are the mainstay of therapy. Although trials are lacking to demonstrate the superiority of any given approach, intravenous immunoglobulin (IVIG) and systemic steroids are frequent initial choices [59, 66]. Studies comparing outcomes after initial treatment using IVIG alone, steroids alone, or a combination of IVIG and steroids have come to differing conclusions on their relative importance in treatment. The combination of both has been reported to lead to faster and more sustained resolution of fever than IVIG alone [67]. Biologic treatments including anakinra, infliximab, or tocilizumab have also been used in refractory cases [66, 68-70], though data are limited to inform the choice among these interventions or those patients who would benefit most. Despite these limitations, overall outcomes of children with MIS-C have been generally good with few fatalities reported [62, 71].
References
1. Centers for Disease Control and Prevention. Demographic Trends of COVID-19 cases and deaths in the US reported to CDC. Available at: https://covid.cdc.gov/covid-data-tracker/#demographics. Accessed 21 November 2021.
2. Stokes EK, Zambrano LD, Anderson KN, et al. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(24): 759-65.
3. Kim L, Whitaker M, O'Halloran A, et al. Hospitalization Rates and Characteristics of Children Aged <18 Years Hospitalized with Laboratory-Confirmed COVID-19 - COVID-NET, 14 States, March 1-July 25, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(32): 1081-8.
4. Han MS, Choi EH, Chang SH, et al. Clinical Characteristics and Viral RNA Detection in Children With Coronavirus Disease 2019 in the Republic of Korea. JAMA Pediatr 2021; 175(1): 73-80.
5. Chung E, Chow EJ, Wilcox NC, et al. Comparison of Symptoms and RNA Levels in Children and Adults With SARS-CoV-2 Infection in the Community Setting. JAMA Pediatr 2021; 175(10): e212025.
6. Delahoy MJ, Ujamaa D, Whitaker M, et al. Hospitalizations Associated with COVID-19 Among Children and Adolescents - COVID-NET, 14 States, March 1, 2020-August 14, 2021. MMWR Morb Mortal Wkly Rep 2021; 70(36): 1255-60.
7. Siegel DA, Reses HE, Cool AJ, et al. Trends in COVID-19 Cases, Emergency Department Visits, and Hospital Admissions Among Children and Adolescents Aged 0-17 Years - United States, August 2020-August 2021. MMWR Morb Mortal Wkly Rep 2021; 70(36): 1249-54.
8. Williams N, Radia T, Harman K, Agrawal P, Cook J, Gupta A. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review of critically unwell children and the association with underlying comorbidities. Eur J Pediatr 2021; 180(3): 689-97.
9. Hurst JH, Heston SM, Chambers HN, et al. Severe Acute Respiratory Syndrome Coronavirus 2 Infections Among Children in the Biospecimens from Respiratory Virus-Exposed Kids (BRAVE Kids) Study. Clin Infect Dis 2021; 73(9): e2875-e82.
10. Paul LA, Daneman N, Schwartz KL, et al. Association of Age and Pediatric Household Transmission of SARS-CoV-2 Infection. JAMA Pediatr 2021; 175(11): 1151-8.
11. Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and Validation of the Pediatric Medical Complexity Algorithm (PMCA) Version 3.0. Acad Pediatr 2018; 18(5): 577-80.
12. Leeb RT, Price S, Sliwa S, et al. COVID-19 Trends Among School-Aged Children - United States, March 1-September 19, 2020. MMWR Morb Mortal Wkly Rep 2020; 69(39): 1410-5.
13. Duarte-Salles T, Vizcaya D, Pistillo A, et al. Thirty-Day Outcomes of Children and Adolescents With COVID-19: An International Experience. Pediatrics 2021; 148(3).
14. Kompaniyets L, Agathis NT, Nelson JM, et al. Underlying Medical Conditions Associated With Severe COVID-19 Illness Among Children. JAMA Netw Open 2021; 4(6): e2111182.
15. WHO Solidarity Trial Consortium, Pan H, Peto R, et al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med 2021; 384(6): 497-511.
16. Goldman JD, Lye DCB, Hui DS, et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N Engl J Med 2020; 383: 1827-37.
17. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med 2020; 383(19): 1813-26.
18. Spinner CD, Gottlieb RL, Criner GJ, et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020; 324(11): 1048-57.
19. Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020; 395(10236): 1569-78.
20. Chiotos K, Hayes M, Kimberlin DW, et al. Multicenter Interim Guidance on Use of Antivirals for Children With Coronavirus Disease 2019/Severe Acute Respiratory Syndrome Coronavirus 2. J Pediatric Infect Dis Soc 2021; 10(1): 34-48.
21. Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate Use of Remdesivir in Children With Severe COVID-19. Pediatrics 2021; 147(5).
22. Gotzinger F, Santiago-Garcia B, Noguera-Julian A, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health 2020; 4(9): 653-61.
23. Gilead Sciences, Inc. Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Efficacy of Remdesivir (GS-5734™) in Participants From Birth to < 18 Years of Age With Coronavirus Disease 2019 (COVID-19) (CARAVAN). Available at: https://www.clinicaltrials.gov/ct2/show/NCT04431453. Accessed 18 November 2020.
24. Ahmed A, Rojo P, Agwu A, et al. Remdesivir Treatment for COVID-19 in Hospitalized Children: CARAVAN Interim Results. In: Conference on Retroviruses and Opportunistic Infections. Virtual, 2022.
25. WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne JAC, Murthy S, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis. JAMA 2020; 324(13): 1330-41.
26. RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021; 384(8): 693-704.
27. REMAP-CAP Investigators, Gordon AC, Mouncey PR, et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med 2021; 384(16): 1491-502.
28. Rosas IO, Brau N, Waters M, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med 2021; 384(16): 1503-16.
29. Hermine O, Mariette X, Tharaux PL, et al. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia: A Randomized Clinical Trial. JAMA Intern Med 2020; 181(1): 32-40.
30. Salama C, Han J, Yau L, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 2021; 384(1): 20-30.
31. Salvarani C, Dolci G, Massari M, et al. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med 2020; 181(1): 24-31.
32. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med 2020; 383: 2333-44.
33. Veiga VC, Prats J, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021; 372: n84.
34. Horby PW, Pessoa-Amorim G, Peto L, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. Lancet 2021; 397(10285): 1637-45.
35. Lescure FX, Honda H, Fowler RA, et al. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med 2021; 9(5): 522-32.
36. Sivapalasingam S, Lederer D, Bhore R, et al. A Randomized Placebo-Controlled Trial of Sarilumab in Hospitalized Patients with Covid-19. medRxiv 2021: Available at: https://doi.org/10.1101/2021.05.13.21256973 [Preprint 14 May 2021].
37. Bhumbra S, Malin S, Kirkpatrick L, et al. Clinical Features of Critical Coronavirus Disease 2019 in Children. Pediatr Crit Care Med 2020; 21(10): e948-e53.
38. Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and Outcomes of US Children and Adolescents With Multisystem Inflammatory Syndrome in Children (MIS-C) Compared With Severe Acute COVID-19. JAMA 2021; 325(11): 1074-87.
39. Garcia-Salido A, de Carlos Vicente JC, Belda Hofheinz S, et al. Severe manifestations of SARS-CoV-2 in children and adolescents: from COVID-19 pneumonia to multisystem inflammatory syndrome: a multicentre study in pediatric intensive care units in Spain. Crit Care 2020; 24(1): 666.
40. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med 2021; 9(12): 1407-18.
41. Marconi VC, Ramanan AV, de Bono S, et al. Baricitinib plus Standard of Care for Hospitalized Adults with COVID-19. medRxiv 2021: Available at: https://doi.org/10.1101/2021.04.30.21255934 [Preprint 3 May 2021].
42. Ely EW, Ramanan AV, Kartman CE, et al. Baricitinib plus Standard of Care for Hospitalised Adults with COVID-19 on Invasive Mechanical Ventilation or Extracorporeal Membrane Oxygenation: Results of a Randomised, Placebo-Controlled Trial. medRxiv 2021: Available at: https://doi.org/10.1101/2021.10.11.21263897 [Preprint 12 October 2021].
43. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med 2021; 384: 795-807.
44. U.S. Food and Drug Administration. Baricitinib EUA Letter of Authorization In: Eli Lilly and Company. Indianapolis, IN: Lilly Corporate Center, 2021.
45. Kim H, Brooks KM, Tang CC, et al. Pharmacokinetics, Pharmacodynamics, and Proposed Dosing of the Oral JAK1 and JAK2 Inhibitor Baricitinib in Pediatric and Young Adult CANDLE and SAVI Patients. Clin Pharmacol Ther 2018; 104(2): 364-73.
46. Eli Lilly and Company. A Study of Baricitinib (LY3009104) in Children With COVID-19 (COV-BARRIER-PEDS) (COV-BARRIER). Available at: https://clinicaltrials.gov/ct2/show/NCT05074420. Accessed 21 November 2021.
47. Guimaraes PO, Quirk D, Furtado RH, et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med 2021; 385(5): 406-15.
48. U.S. Food and Drug Administration. Highlights of Prescribing Information: XELJANZ® (tofacitinib) (package insert). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/213082s000lbl.pdf. Accessed 11 August 2022.
49. Hammond J, Leister-Tebbe H, Gardner A, et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19. N Engl J Med 2022; 386(15): 1397-408.
50. Belhadjer Z, Meot M, Bajolle F, et al. Acute Heart Failure in Multisystem Inflammatory Syndrome in Children in the Context of Global SARS-CoV-2 Pandemic. Circulation 2020; 142(5): 429-36.
51. Deza Leon MP, Redzepi A, McGrath E, et al. COVID-19-Associated Pediatric Multisystem Inflammatory Syndrome. J Pediatric Infect Dis Soc 2020; 9(3): 407-8.
52. Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet (London, England) 2020; 395(10237): 1607-8.
53. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. The Lancet 2020; 395(10239): 1771-8.
54. Blondiaux E, Parisot P, Redheuil A, et al. Cardiac MRI of Children with Multisystem Inflammatory Syndrome (MIS-C) Associated with COVID-19: Case Series. Radiology 2020: 202288.
55. Greene AG, Saleh M, Roseman E, Sinert R. Toxic shock-like syndrome and COVID-19: A case report of multisystem inflammatory syndrome in children (MIS-C). Am J Emerg Med 2020.
56. Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020; 79(8): 999-1006.
57. Chiotos K, Bassiri H, Behrens EM, et al. Multisystem Inflammatory Syndrome in Children During the Coronavirus 2019 Pandemic: A Case Series. J Pediatric Infect Dis Soc 2020; 9(3): 393-8.
58. Cabrero-Hernandez M, Garcia-Salido A, Leoz-Gordillo I, et al. Severe SARS-CoV-2 Infection in Children With Suspected Acute Abdomen: A Case Series From a Tertiary Hospital in Spain. Pediatr Infect Dis J 2020; 39(8): e195-e8.
59. Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med 2020; 383(4): 334-46.
60. Dufort EM, Koumans EH, Chow EJ, et al. Multisystem Inflammatory Syndrome in Children in New York State. New England Journal of Medicine 2020; 383(4): 347-58.
61. Whittaker E, Bamford A, Kenny J, et al. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA 2020; 324(3): 259-69.
62. Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-Associated Multisystem Inflammatory Syndrome in Children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep 2020; 69(32): 1074-80.
63. Payne AB, Gilani Z, Godfred-Cato S, et al. Incidence of Multisystem Inflammatory Syndrome in Children Among US Persons Infected With SARS-CoV-2. JAMA Netw Open 2021; 4(6): e2116420.
64. Stierman B, Abrams JY, Godfred-Cato SE, et al. Racial and Ethnic Disparities in Multisystem Inflammatory Syndrome in Children in the United States, March 2020 to February 2021. Pediatr Infect Dis J 2021; 40(11): e400-e6.
65. Belay ED, Abrams J, Oster ME, et al. Trends in Geographic and Temporal Distribution of US Children With Multisystem Inflammatory Syndrome During the COVID-19 Pandemic. JAMA Pediatr 2021; 175(8): 837-45.
66. Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2021; 5(2): 133-41.
67. Ouldali N, Toubiana J, Antona D, et al. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone With Course of Fever in Multisystem Inflammatory Syndrome in Children. JAMA 2021; 325(9): 855-64.
68. Kaushik S, Aydin SI, Derespina KR, et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J Pediatr 2020; 224: 24-9.
69. Capone CA, Subramony A, Sweberg T, et al. Characteristics, Cardiac Involvement, and Outcomes of Multisystem Inflammatory Syndrome of Childhood Associated with severe acute respiratory syndrome coronavirus 2 Infection. J Pediatr 2020; 224: 141-5.
70. Celikel E, Tekin ZE, Aydin F, et al. Role of Biological Agents in the Treatment of SARS-CoV-2-Associated Multisystem Inflammatory Syndrome in Children. J Clin Rheumatol 2022; 28(2): e381-e7.
71. Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Available at: https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance. Accessed 28 November 2021.
Section last reviewed and updated 12/23/2020
Last literature search conducted 12/14/2020
Recommendation 1:
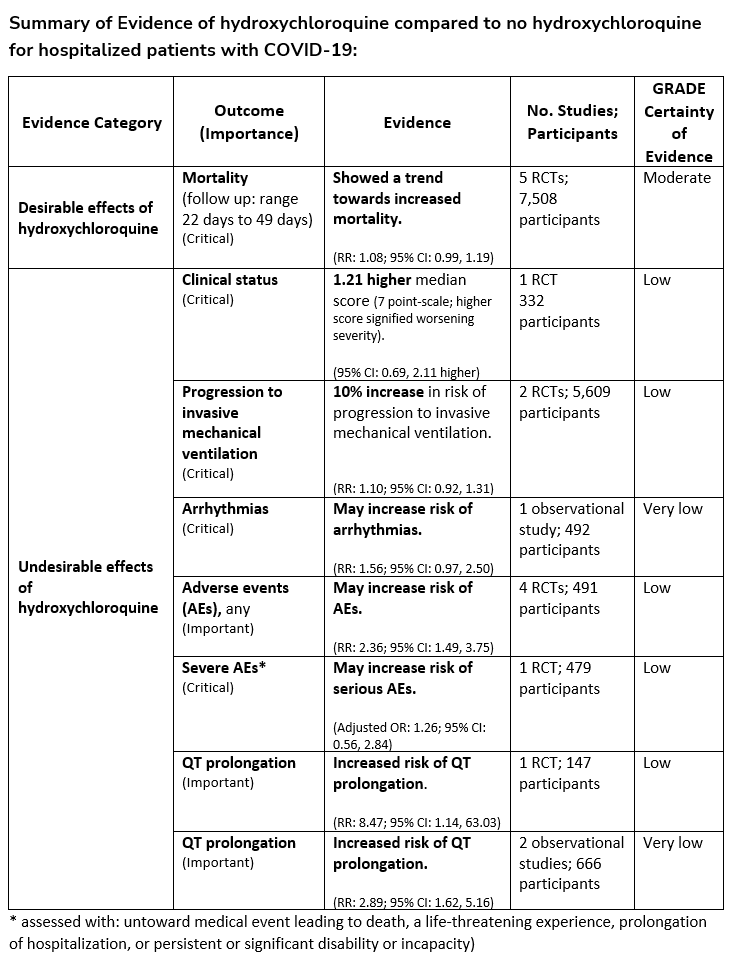
Among hospitalized patients with COVID-19, the IDSA guideline panel recommends against hydroxychloroquine*. (Strong recommendation, Moderate certainty of evidence)
-
Remark: Chloroquine is considered to be class equivalent to hydroxychloroquine.
Recommendation 2:
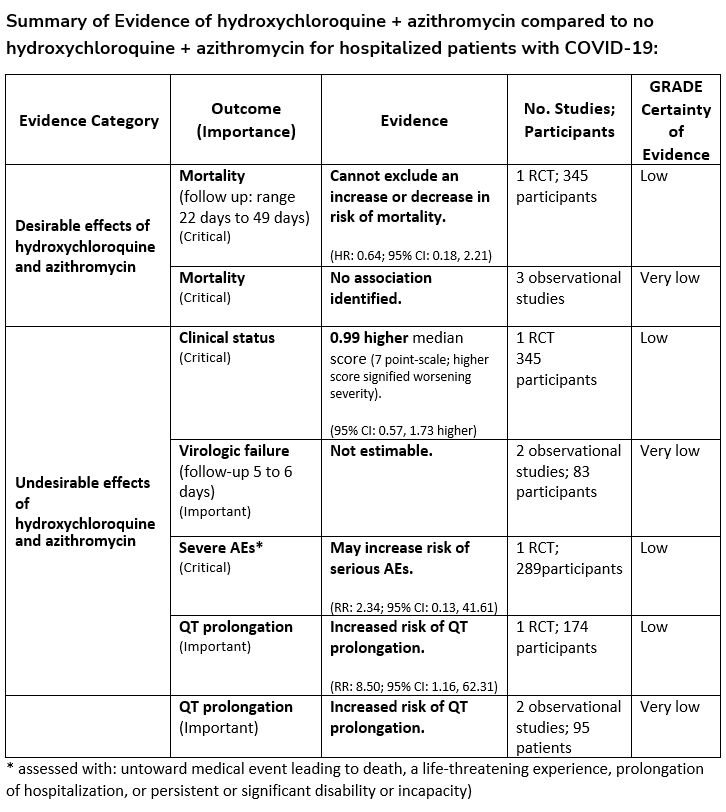
Among hospitalized patients with COVID-19, the IDSA guideline panel recommends against hydroxychloroquine* plus azithromycin. (Strong recommendation, Low certainty of evidence)
- Remark: Chloroquine is considered to be class equivalent to hydroxychloroquine.
Section last reviewed and updated 9/23/2021
Last literature search conducted 9/21/2021
Recommendation 3:
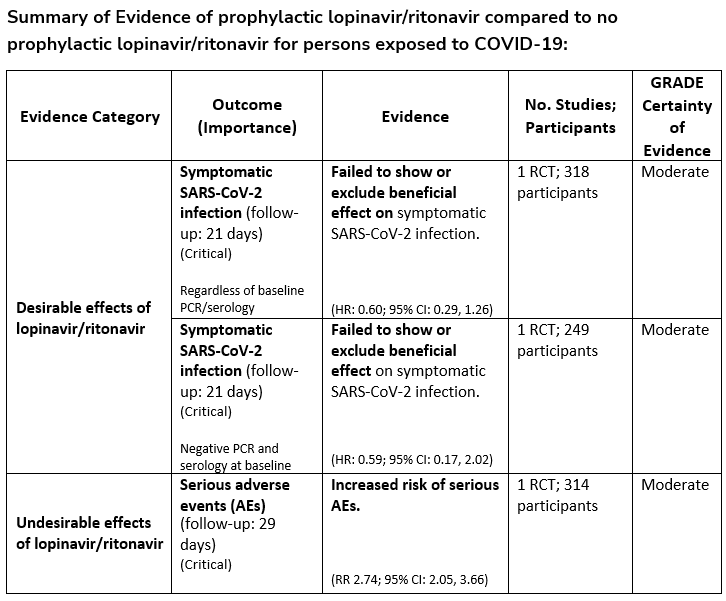
In persons exposed to COVID-19, the IDSA guideline panel recommends against hydroxychloroquine. (Strong recommendation, Low certainty of evidence)
Section last reviewed and updated 2/16/2022
Last literature search conducted 1/31/2022
Recommendation 4:
In persons exposed to COVID-19, the IDSA guideline panel recommends against post-exposure prophylaxis with lopinavir/ritonavir. (Strong recommendation, Moderate certainty of evidence)
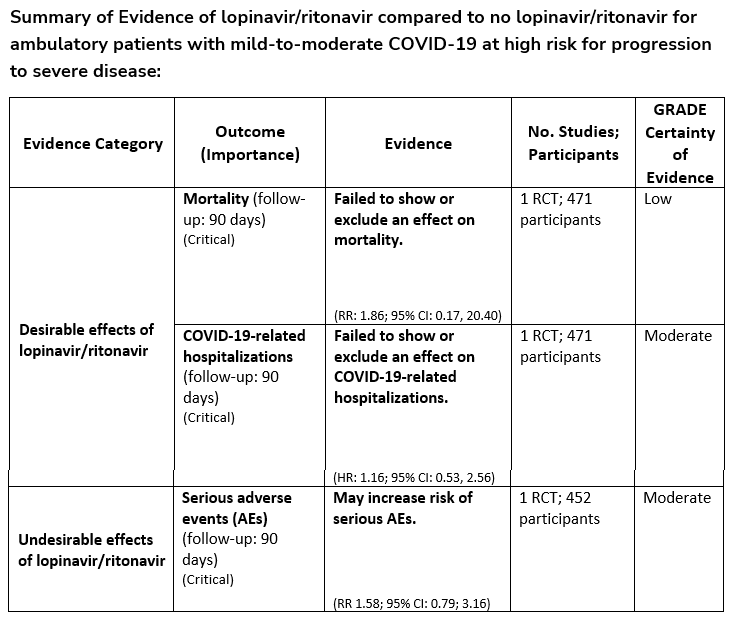
Recommendation 5:
Among ambulatory patients with mild-to-moderate COVID-19, the IDSA guideline panel recommends against the use of lopinavir/ritonavir. (Strong recommendation, Moderate certainty of evidence)
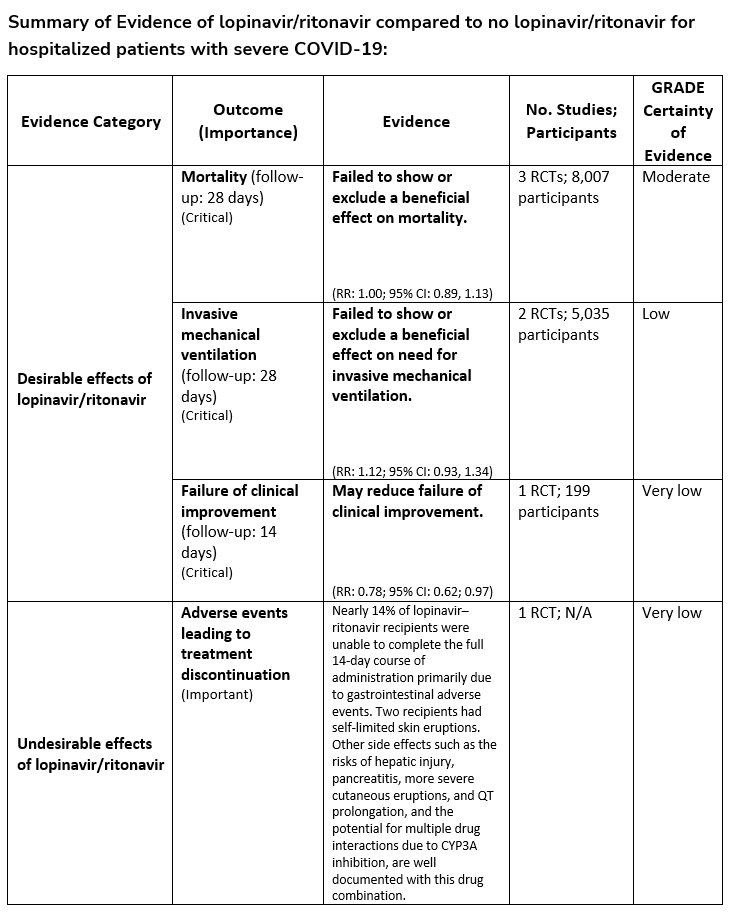
Recommendation 6:
Among hospitalized patients with COVID-19, the IDSA guideline panel recommends against the use of the combination lopinavir/ritonavir. (Strong recommendation, Moderate certainty of evidence)
Section last reviewed and updated 9/25/2020
Last literature search conducted 9/4/2020
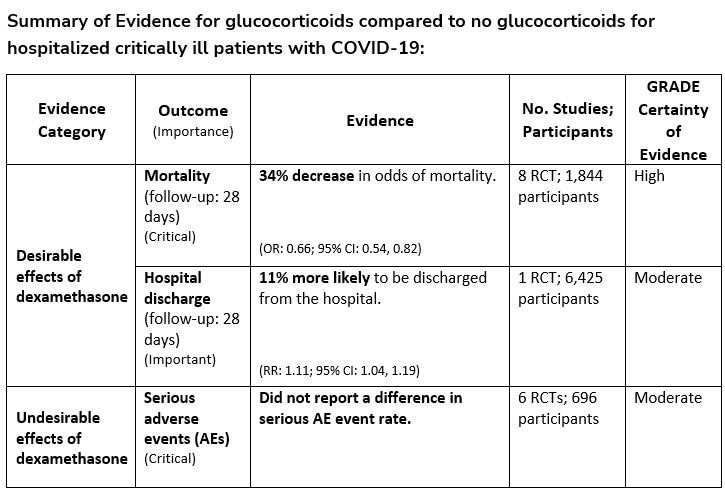
Recommendation 7:
Among hospitalized critically ill patients* with COVID-19, the IDSA guideline panel recommends dexamethasone rather than no dexamethasone. (Strong recommendation, Moderate certainty of evidence)
*Critical illness is defined as patients on mechanical ventilation and ECMO. Critical illness includes end organ dysfunction as is seen in sepsis/septic shock. In COVID-19, the most commonly reported form of end organ dysfunction is ARDS
- Remark: If dexamethasone is unavailable, equivalent total daily doses of alternative glucocorticoids may be used. Dexamethasone 6 mg IV or PO for 10 days (or until discharge) or equivalent glucocorticoid dose may be substituted if dexamethasone unavailable. Equivalent total daily doses of alternative glucocorticoids to dexamethasone 6 mg daily are methylprednisolone 32 mg and prednisone 40 mg.
Recommendation 8:
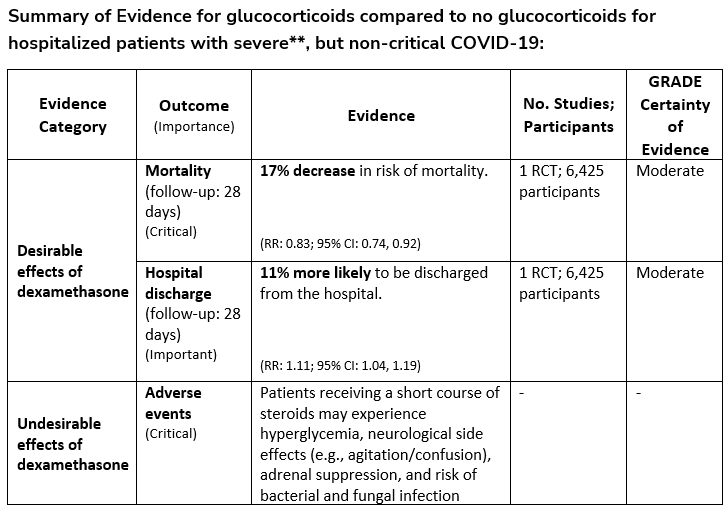
Among hospitalized patients with severe**, but non-critical, COVID-19, the IDSA guideline panel suggests dexamethasone rather than no dexamethasone. (Conditional recommendation†, Moderate certainty of evidence)
**Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen.
- Remark: Dexamethasone 6 mg IV or PO for 10 days (or until discharge) or equivalent glucocorticoid dose may be substituted if dexamethasone unavailable. Equivalent total daily doses of alternative glucocorticoids to dexamethasone 6 mg daily are methylprednisolone 32 mg and prednisone 40 mg.
Recommendation 9:
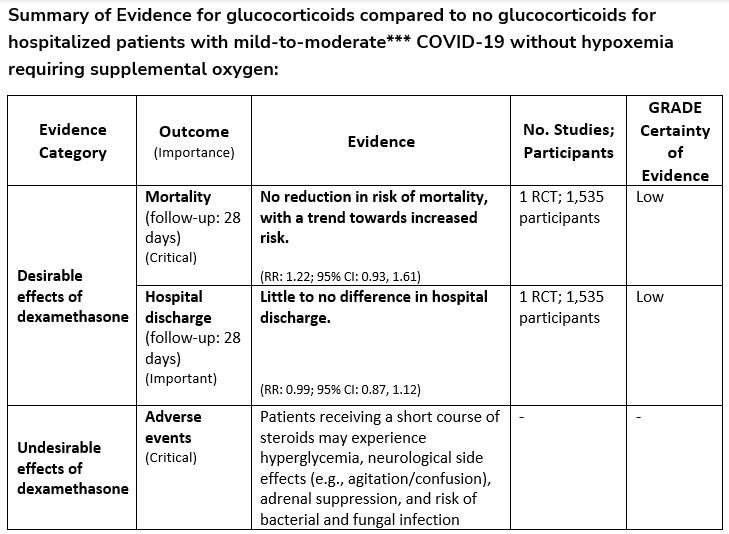
Among hospitalized patients with mild-to-moderate*** COVID-19 without hypoxemia requiring supplemental oxygen, the IDSA guideline panel suggests against the use of glucocorticoids. (Conditional recommendation††, Low certainty of evidence)
***Mild-to-moderate illness is defined as patient with a SpO2 >94% not requiring supplemental oxygen.
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated 10/10/2022
Last literature search conducted 8/31/2022
Recommendation 10:
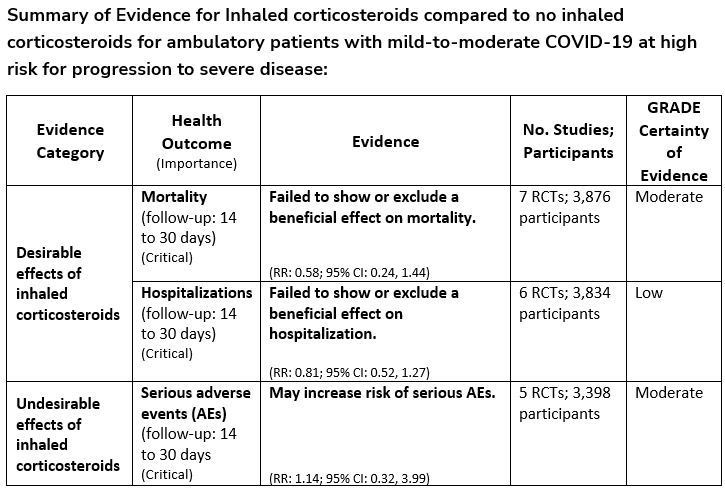
Among ambulatory patients with mild-to-moderate COVID-19, the IDSA guideline panel suggests against inhaled corticosteroids. (Conditional recommendation††, Moderate certainty of evidence)
-
Remark: Patients who are on inhaled corticosteroids for other indications may continue them.
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated on 9/14/2021
Last literature search conducted 8/31/2021
Recommendation 11:
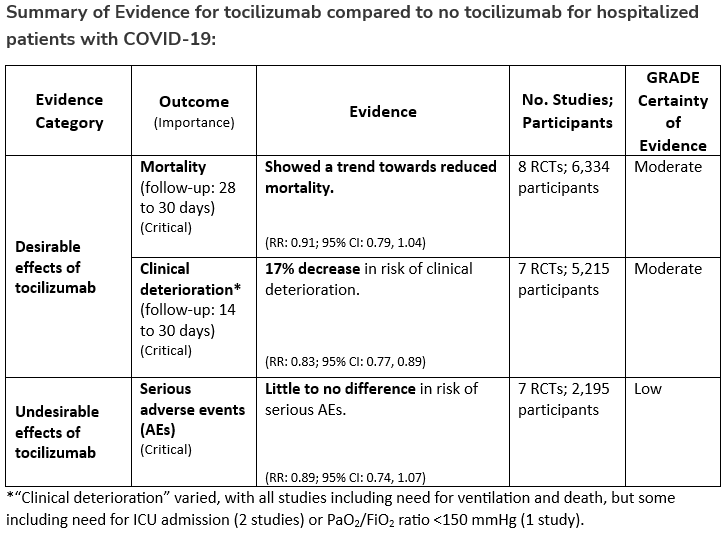
Among hospitalized adults with progressive severe* or critical** COVID-19 who have elevated markers of systemic inflammation, the IDSA guideline panel suggests tocilizumab in addition to standard of care (i.e., steroids) rather than standard of care alone. (Conditional recommendation†, Low certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen.
**Critical illness is defined as patients on mechanical ventilation and ECMO. Critical illness includes end organ dysfunction as is seen in sepsis/septic shock. In COVID-19, the most commonly reported form of end organ dysfunction is ARDS.
- Remarks:
- Patients, particularly those who respond to steroids alone, who put a high value on avoiding possible adverse events of tocilizumab and a low value on the uncertain mortality reduction, would reasonably decline tocilizumab.
- In the largest trial on the treatment of tocilizumab, criterion for systemic inflammation was defined as CRP ≥75 mg/L.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Recommendation 12:
When tocilizumab is not available for patients who would otherwise qualify for tocilizumab, the IDSA guideline panel suggests sarilumab in addition to standard of care (i.e., steroids) rather than standard of care alone. (Conditional recommendation†, Very low certainty of evidence)
- Remark: Patients, particularly those who respond to steroids alone, who put a high value on avoiding possible adverse events of sarilumab and a low value on the uncertain mortality reduction, would reasonably decline sarilumab.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated 2/22/2023
Last literature search conducted 1/31/2023
Recommendation 13:
Among immunocompetent patients hospitalized with COVID-19, the IDSA guideline panel recommends against COVID-19 convalescent plasma. (Strong recommendation, Moderate certainty of evidence)
- Remark: Patients, particularly those who do not qualify for other treatments, who place a higher value on the uncertain mortality reduction and a lower value on the potential adverse effects of convalescent plasma would reasonably select convalescent plasma.
Recommendation 14:
Among immunocompromised patients hospitalized with COVID-19, the IDSA guideline panel suggests against the routine use of COVID-19 convalescent plasma. (Conditional recommendation, very low certainty of evidence)
- Remark: Patients, particularly those who do not qualify for other treatments, who place a higher value on the uncertain mortality reduction and a lower value on the potential adverse effects of convalescent plasma would reasonably select convalescent plasma.
Recommendation 15:
Among ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease who have no other treatment options*, the IDSA guideline panel suggests FDA-qualified high-titer COVID-19 convalescent plasma within 8 days of symptom onset rather than no high-titer COVID-19 convalescent plasma. (Conditional recommendation†, Low certainty of evidence)
*Other options for treatment and management of ambulatory patients include nirmatrelvir/ritonavir and three-day treatment with remdesivir. Patient-specific factors (e.g., symptom duration, renal insufficiency or other contraindications, drug interactions) as well as logistical challenges, infusion capacity, and product availability should drive decision-making regarding choice of agent. Data for combination treatment do not exist in this setting.
- Remarks:
- In the United States, FDA emergency use authorization (EUA) only authorizes use in patients with immunosuppressive disease or receiving immunosuppressive treatment.
- Patients, particularly those who are not immunocompromised, who place a low value on the uncertain benefits (reduction in the need for mechanical ventilation, hospitalization, and death) and a high value on avoiding possible adverse events associated with convalescent plasma would reasonably decline convalescent plasma.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
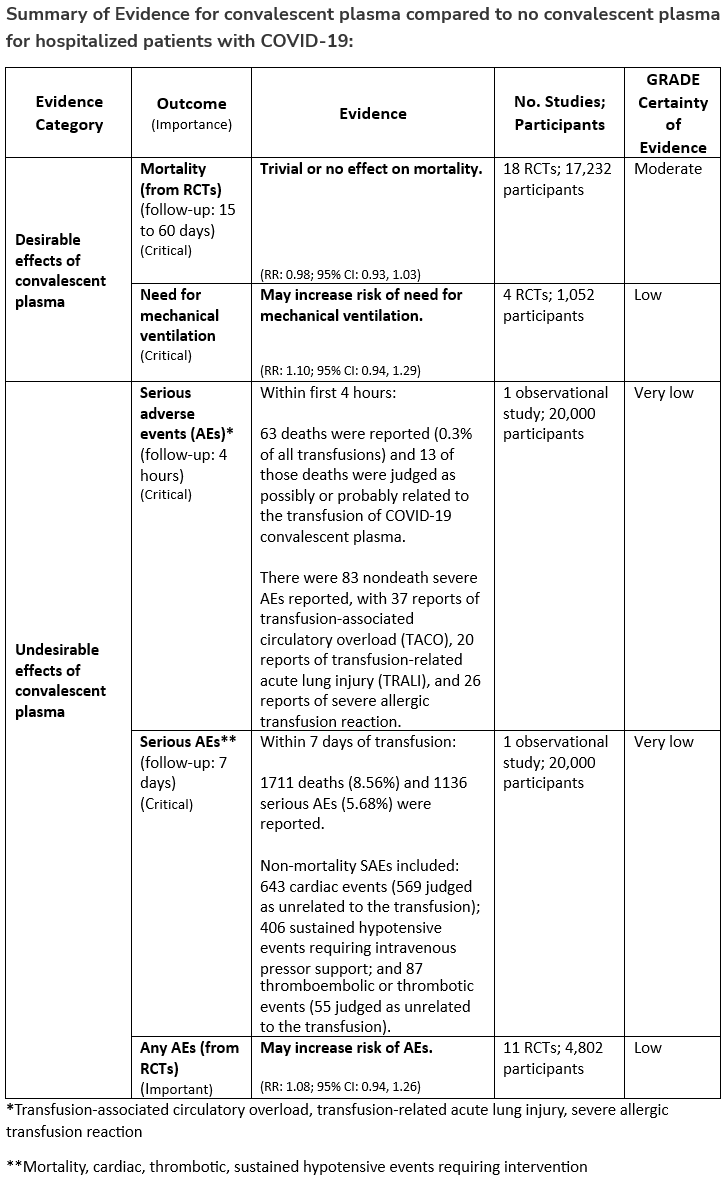
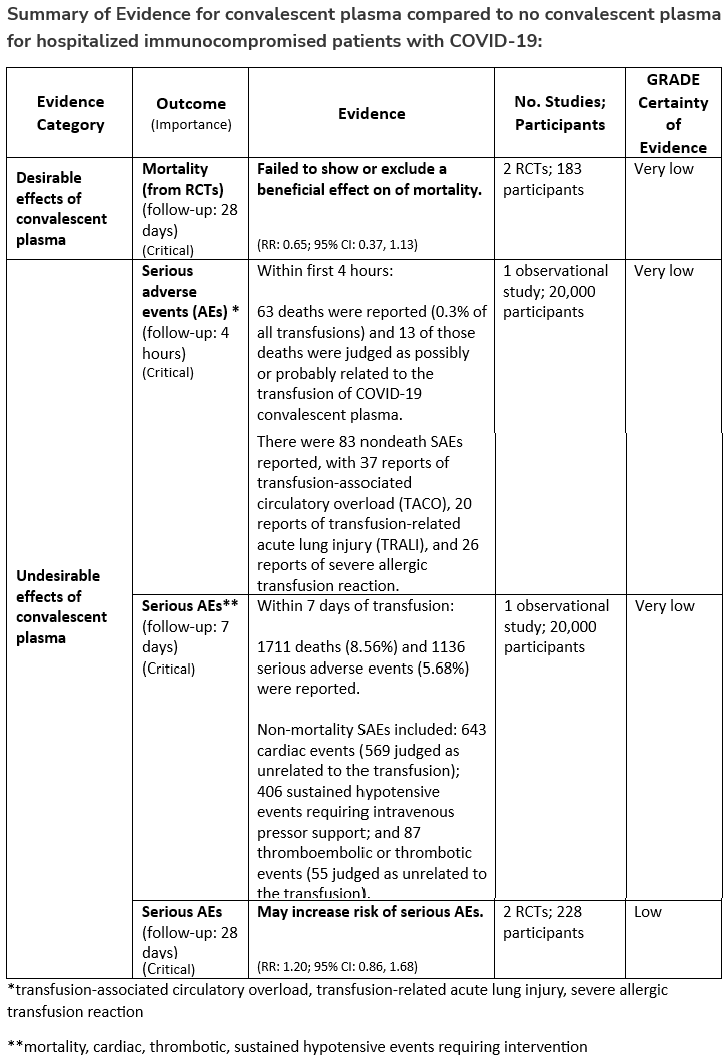
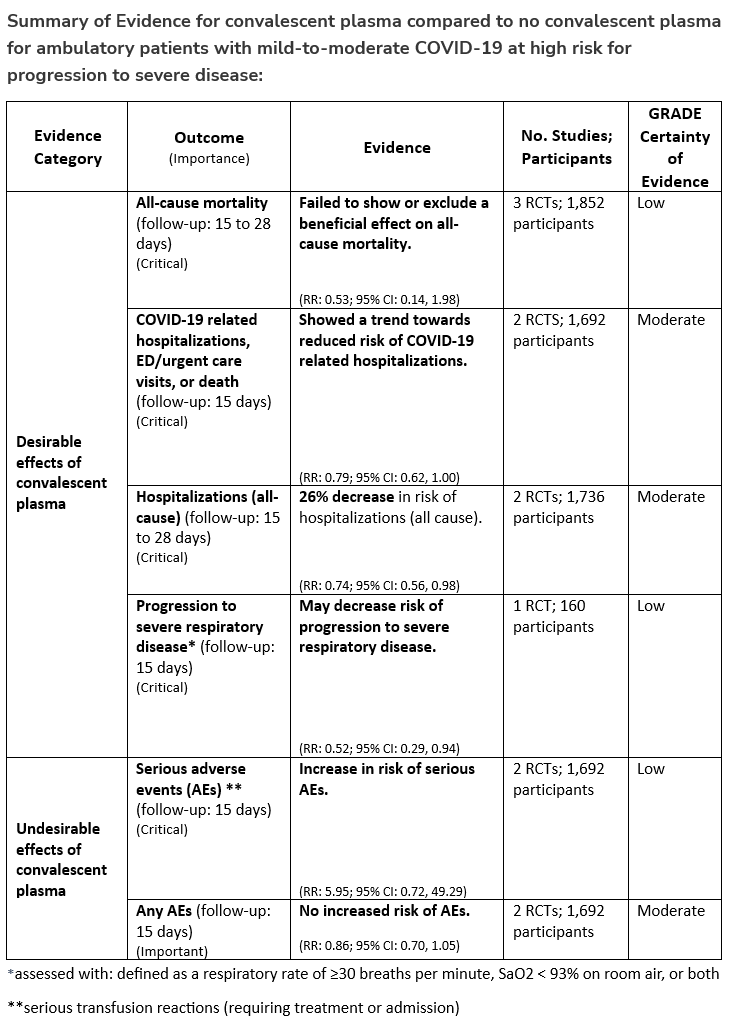
Section last reviewed and updated 2/7/2022
Last literature search conducted 1/31/2022
Recommendation 16:
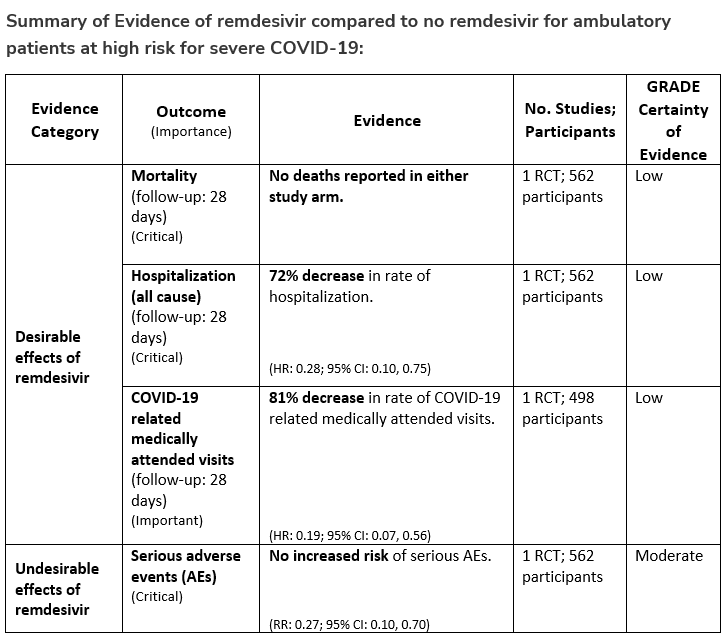
Among patients (ambulatory or hospitalized) with mild-to-moderate COVID-19 at high risk for progression to severe disease, the IDSA guideline panel suggests remdesivir initiated within seven days of symptom onset rather than no remdesivir. (Conditional recommendation†, Low certainty of evidence)
- Remarks:
- Dosing for remdesivir in mild-to-moderate COVID-19 is 200 mg on day one followed by 100 mg on days two and three. Pediatric dosing is 5 mg/kg on day 1 and 2.5 mg/kg on subsequent days.
- Options for treatment and management of ambulatory patients include nirmatrelvir/ritonavir, three-day treatment with remdesivir, molnupiravir, and neutralizing monoclonal antibodies. Patient-specific factors (e.g., patient age, symptom duration, renal function, drug interactions), product availability, and institutional capacity and infrastructure should drive decision-making regarding choice of agent. Data for combination treatment do not exist in this setting.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
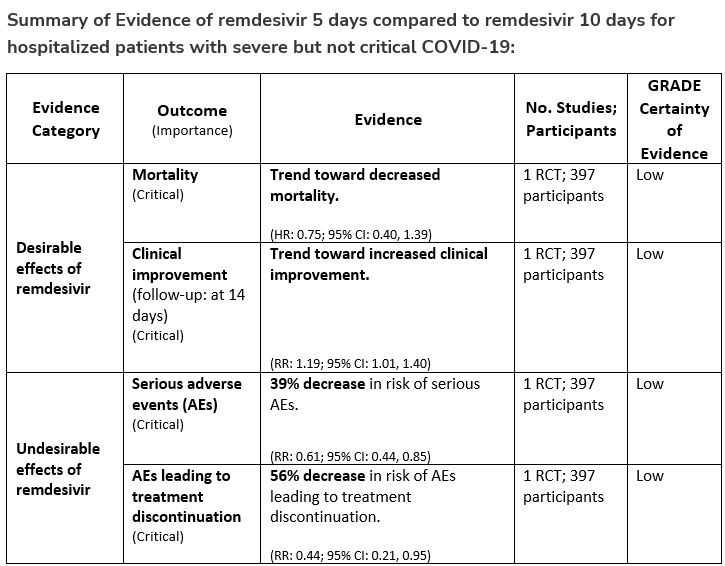
Recommendation 17:
In patients on supplemental oxygen but not on mechanical ventilation or ECMO, the IDSA panel suggests treatment with five days of remdesivir rather than 10 days of remdesivir. (Conditional recommendation†, Low certainty of evidence)
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
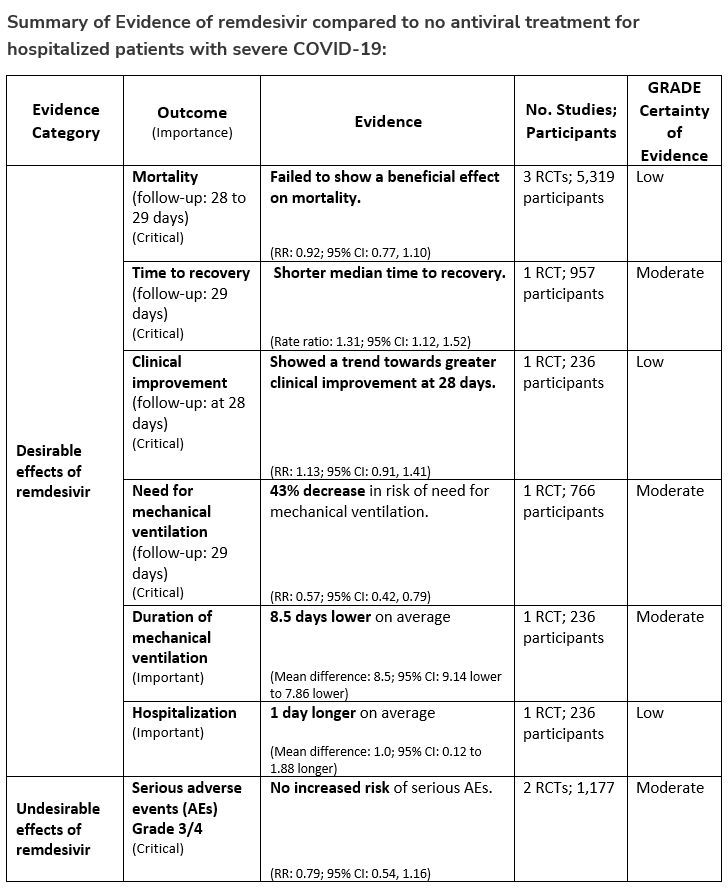
Recommendation 18a:
In hospitalized patients with severe* COVID-19, the IDSA panel suggests remdesivir over no antiviral treatment. (Conditional recommendation†, Moderate certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
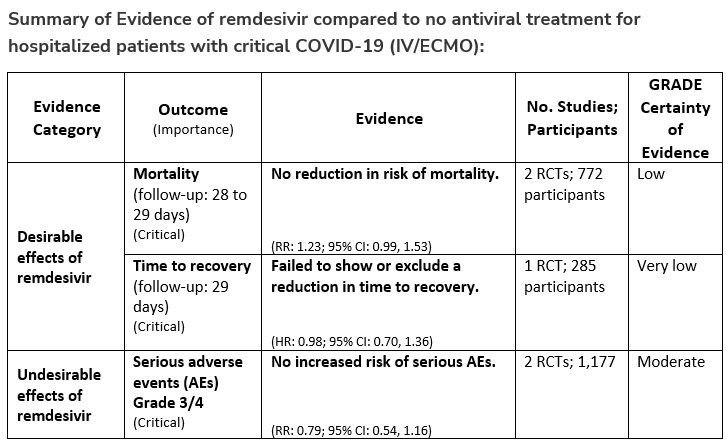
Recommendation 18b:
In patients with COVID-19 on invasive ventilation and/or ECMO, the IDSA panel suggests against the routine initiation of remdesivir (Conditional recommendation††, Very low certainty of evidence)
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated 5/23/2022
Last literature search conducted 4/30/2022
Recommendation 19:
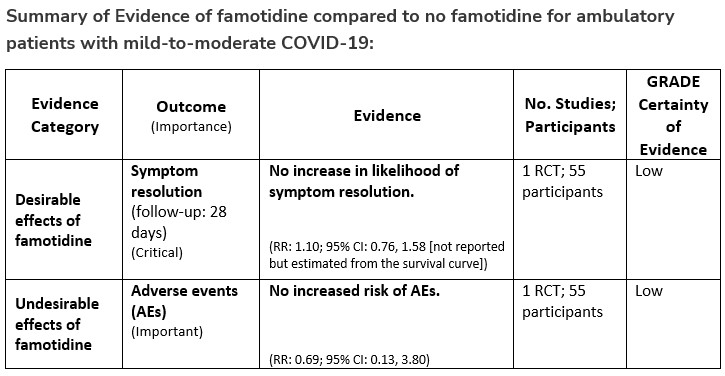
Among ambulatory patients with mild-to-moderate COVID-19, the IDSA panel suggests against famotidine for the treatment of COVID-19 (Conditional recommendation††, Low certainty of evidence)
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Recommendation 20:
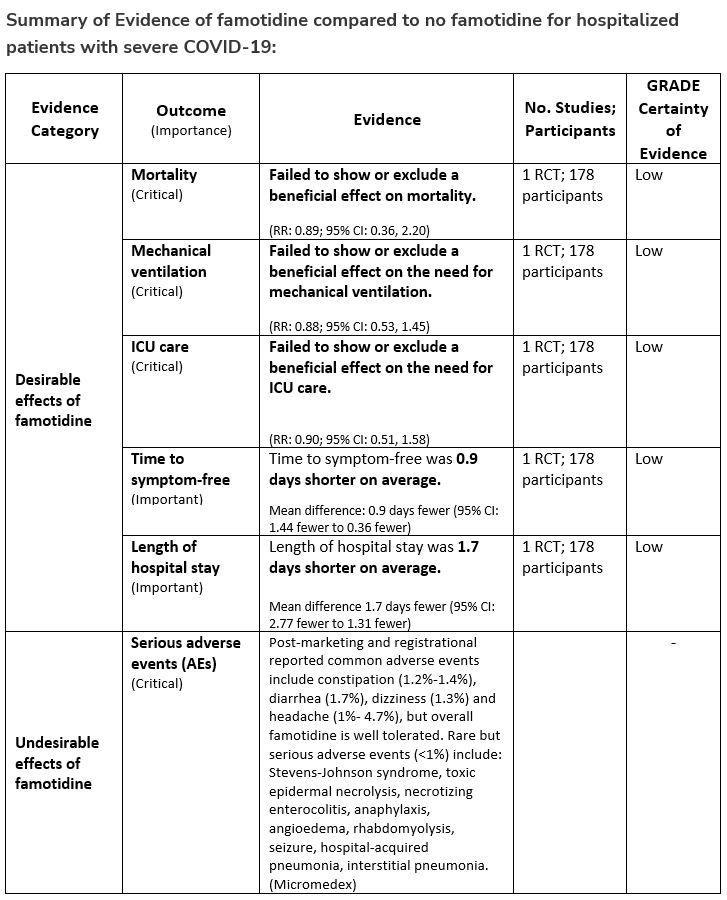
Among hospitalized patients with severe* COVID-19, the IDSA panel suggests against famotidine for the treatment of COVID-19. (Conditional recommendation††, Low certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen.
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated on 1/27/2023
As of 1/26/2023, based on CDC Nowcast data, fewer than 10% of circulating variants in the US are susceptible to tixagevimab/cilgavimab (Evusheld), the sole product that has been available for pre-exposure prophylaxis. Tixagevimab/cilgavimab is therefore no longer authorized for use in the US until further notice by FDA.
SARS-CoV-2 is expected to continue to evolve. Although the general trend has been towards increasing resistance to neutralizing monoclonal antibodies, there have been instances in which new variants became more susceptible to existing anti-SARS CoV-2 neutralizing antibodies. Should this occur again, or should newly developed, more active neutralizing antibodies be authorized for prophylaxis, the panel will offer recommendations regarding use.
Please see the retired versions of this section below:
Section last reviewed and updated on 1/12/2023
As the pandemic progressed, new SARS CoV-2 variants emerged with reduced susceptibility to various anti-SARS-CoV-2 neutralizing antibodies in assays performed using infectious (also referred to as authentic) and pseudotyped viruses. The first two US FDA authorized anti-SARS-CoV-2 neutralizing antibody combinations, bamlanivimab/etesevimab and casirivimab/imdevimab, were found to be largely inactive against the Omicron BA.1 and BA.2 variants, rendering these products no longer useful for either treatment or post-exposure prophylaxis. As a result, Emergency Use Authorization was withdrawn by the US FDA for both bamlanivimab/etesevimab and casirivimab/imdevimab, leaving no available neutralizing antibody product for use in the United States for post-exposure prophylaxis. Should new variants become susceptible to an existing neutralizing antibody or should newly developed, more susceptible neutralizing antibodies be authorized for post-exposure prophylaxis, the panel will offer recommendations regarding use.
For areas of the world where a significant proportion of circulating variants retain susceptibility to at least one neutralizing antibody authorized for post-exposure prophylaxis, use could be considered. However, data are scarce on how susceptibility reductions affect clinical efficacy, relative to that observed prior to emergence of novel variants.
Section last reviewed and updated 1/12/2023
During 2022, multiple Omicron sub-variants with progressively greater in vitro reductions in susceptibility to multiple anti-SARS CoV-2 neutralizing antibodies emerged. On November 30, 2022, the US FDA withdrew Emergency Use Authorization for bebtelovimab, the one anti-SARS CoV-2 neutralizing antibody product that had retained in vitro activity against most previously circulating SARS-CoV-2 variants, leaving no available neutralizing antibody product in the United States for treatment of COVID-19.
For areas of the world where a significant proportion of circulating variants retain susceptibility to at least one authorized therapeutic neutralizing antibody, use could be considered, taking into account the predicted relative benefits of the anti-SARS CoV-2 neutralizing antibody product compared with alternative antiviral therapies. However, data are scarce on how susceptibility reductions affect clinical efficacy, relative to that observed prior to emergence of novel variants.
SARS-CoV-2 is expected to continue to evolve. Although the general trend has been towards increasing resistance to therapeutic neutralizing monoclonal antibodies, there have been instances in which new variants became more susceptible to existing anti-SARS CoV-2 neutralizing antibodies. Should this occur again, or should newly developed, more active neutralizing antibodies be authorized for treatment, the panel will offer recommendations regarding use.
Please see the retired version of this section below:
Baricitinib
Section last reviewed and updated 4/29/2022
Last literature search conducted 3/31/2022
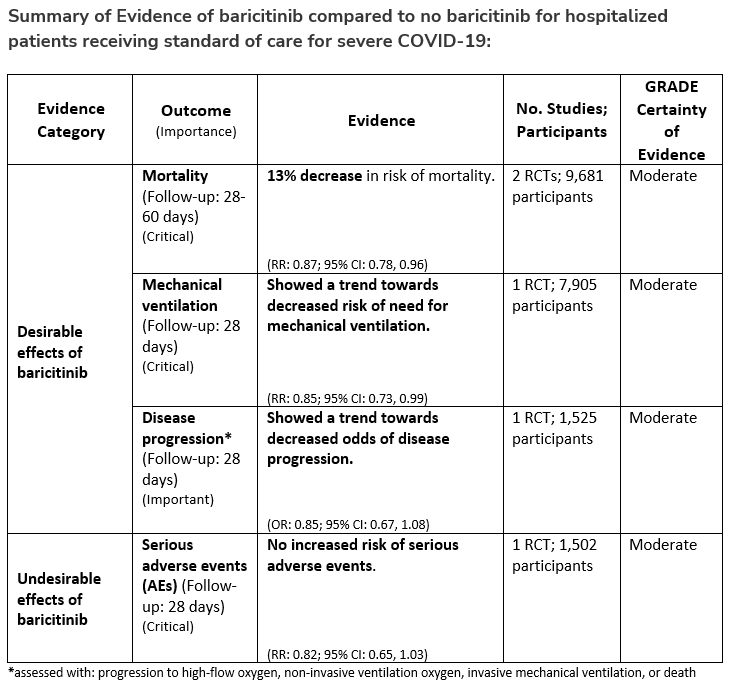
Recommendation 21:
Among hospitalized adults with severe* COVID-19, the IDSA panel suggests baricitinib with corticosteroids rather than no baricitinib. (Conditional recommendation†, Moderate certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen, oxygen through a high-flow device, or non-invasive ventilation.
- Remarks:
- Baricitinib 4 mg per day (or appropriate renal dosing) up to 14 days or until discharge from hospital.
- Baricitinib appears to demonstrate the most benefit in those with severe COVID-19 on high-flow oxygen/non-invasive ventilation at baseline.
- Limited additional data suggest a mortality reduction even among patients requiring mechanical ventilation.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Recommendation 22:
Among hospitalized patients with severe* COVID-19 who cannot receive a corticosteroid (which is standard of care) because of a contraindication, the IDSA guideline panel suggests use of baricitinib with remdesivir rather than remdesivir alone. (Conditional recommendation†, Low certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen, oxygen through a high-flow device, or non-invasive ventilation.
- Remark:
- Baricitinib 4 mg daily dose for 14 days or until hospital discharge. The benefits of baricitinib plus remdesivir for persons on mechanical ventilation are uncertain.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Tofacitinib
Section last reviewed and updated 8/21/2021
Last literature search conducted 7/31/2021
Recommendation 23:
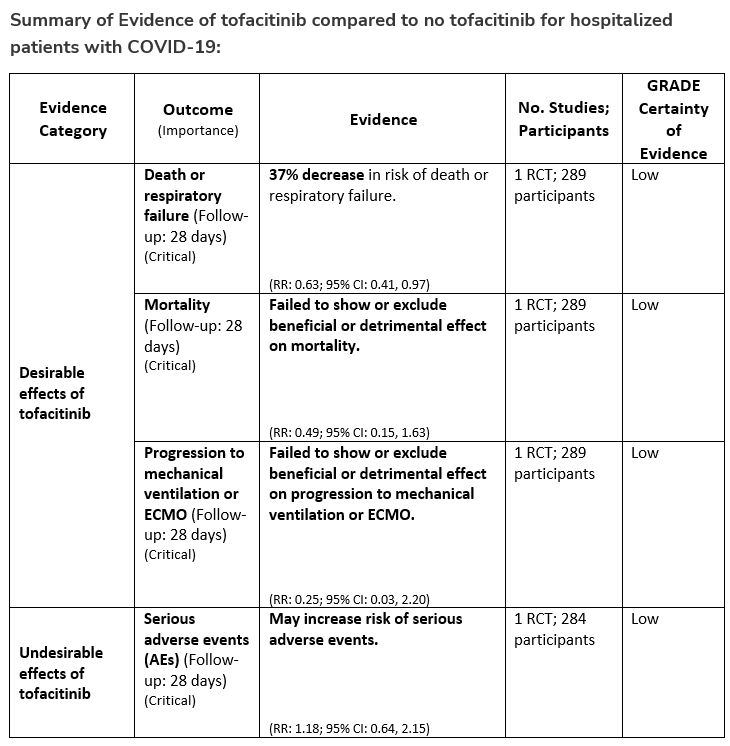
Among hospitalized adults with severe* COVID-19 but not on non-invasive or invasive mechanical ventilation, the IDSA panel suggests tofacitinib rather than no tofacitinib. (Conditional recommendation†, Low certainty of evidence)
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen or oxygen through a high-flow device.
- Remarks:
- Tofacitinib appears to demonstrate the most benefit in those with severe COVID-19 on supplemental or high-flow oxygen.
- Patients treated with tofacitinib should be on at least prophylactic dose anticoagulant.
- Patients who receive tofacitinib should not receive tocilizumab or other IL-6 inhibitor for treatment of COVID-19.
- The STOP-COVID Trial did not include immunocompromised patients.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated 10/10/2022
Last literature search conducted 8/31/2022
Recommendation 24:
In hospitalized patients with COVID-19, the IDSA panel suggests against ivermectin. (Conditional recommendation††, Very low certainty of evidence)
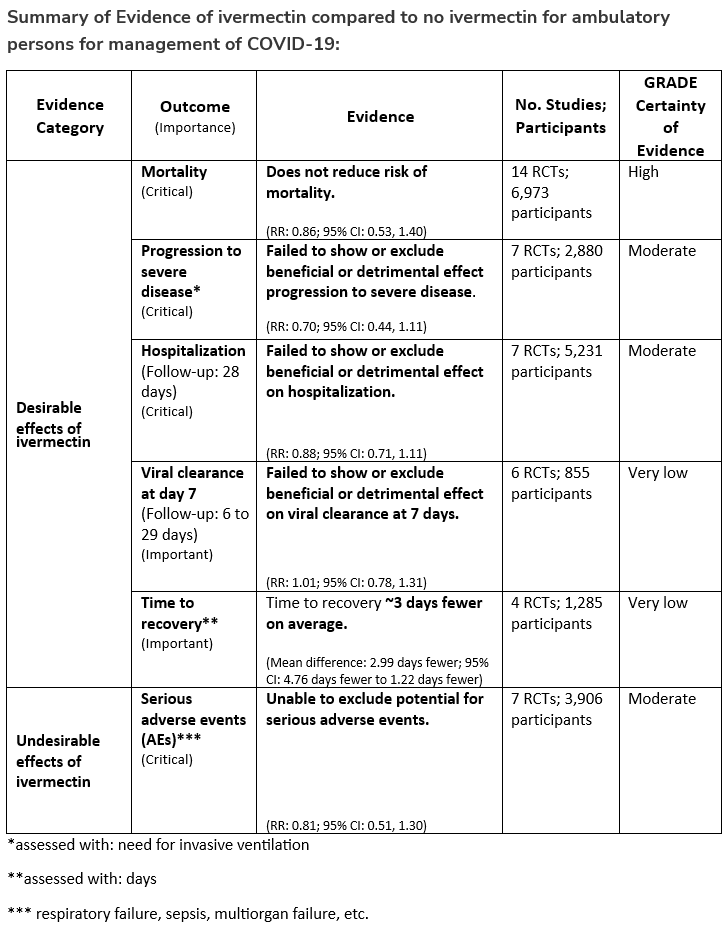
Recommendation 25:
In ambulatory persons with COVID-19, the IDSA panel recommends against ivermectin. (Strong recommendation, Moderate certainty of evidence)
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
Section last reviewed and updated 11/8/2021
Last literature search conducted 10/31/2021
Recommendation 26:
Among ambulatory patients with COVID-19, the IDSA guideline panel recommends fluvoxamine only in the context of a clinical trial. (Knowledge gap)
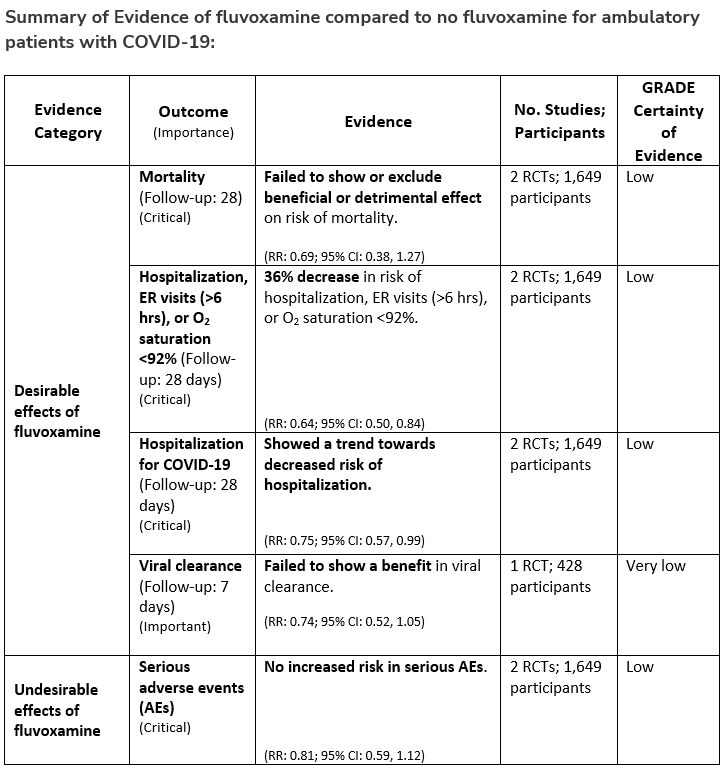
Section last reviewed and updated 2/7/2022
Last literature search conducted 1/31/2022
Recommendation 27:
In ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease, the IDSA guideline panel suggests nirmatrelvir/ritonavir initiated within five days of symptom onset rather than no nirmatrelvir/ritonavir. (Conditional recommendation†, Low certainty of evidence)
- Remarks:
- Patients’ medications need to be screened for serious drug interactions
Dosing based on renal function:- Estimated glomerular filtration rate (eGFR) > 60 ml/min: 300 mg nirmatrelvir/100 ritonavir every 12 hours for five days
- eGFR ≤60 mL/min and ≥30 mL/min: 150 mg nirmatrelvir/100 mg ritonavir every 12 hours for five days
- eGFR <30 mL/min: not recommended
- Patients with mild-to-moderate COVID-19 who are at high risk of progression to severe disease admitted to the hospital may also receive nirmatrelvir/ritonavir
- Patients’ medications need to be screened for serious drug interactions
*Options for treatment and management of ambulatory patients include nirmatrelvir/ritonavir, remdesivir for a 3-day course, molnupiravir, and neutralizing monoclonal antibodies. Patient-specific factors (e.g., symptom duration, renal function, drug interactions) as well as product availability should drive decision-making regarding choice of agent. Data for combination treatment do not exist in this setting.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
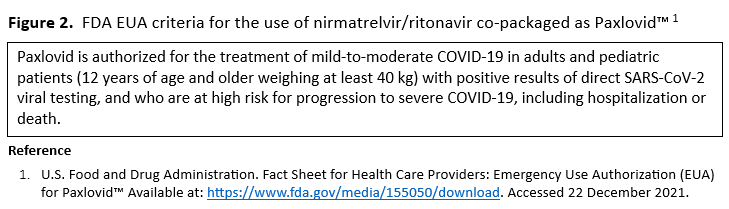
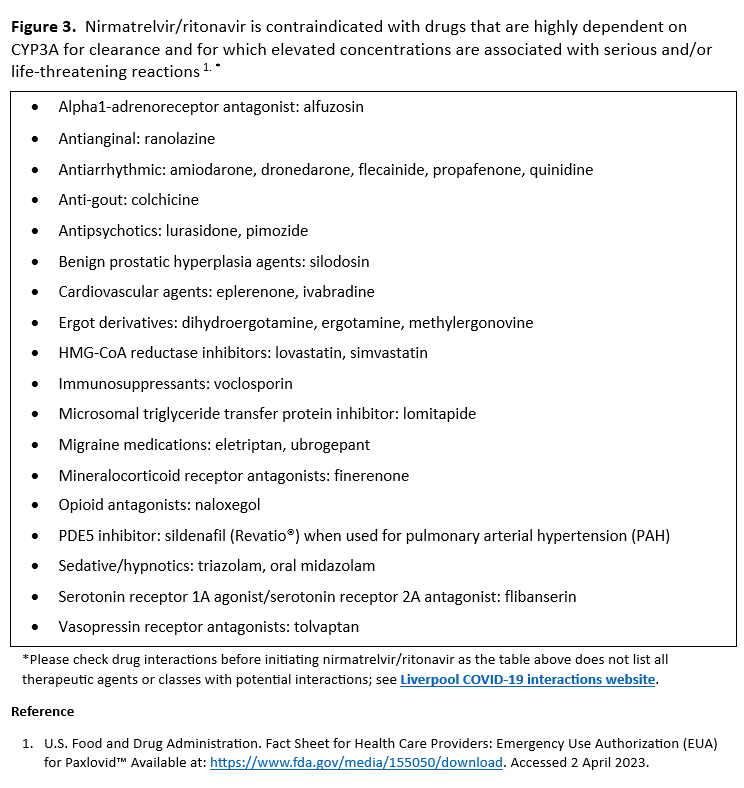

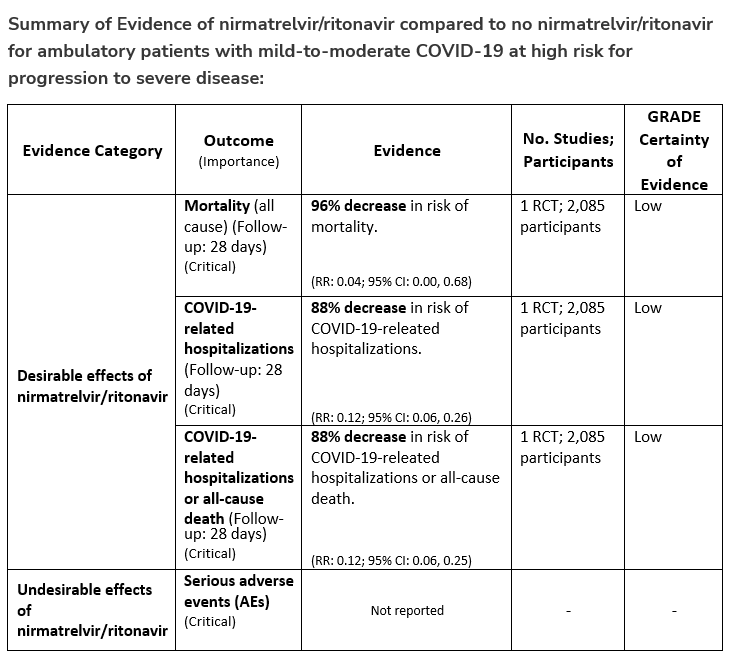
Section last reviewed and updated 2/23/2023
Last literature search conducted 1/31/2023
Recommendation 28:
In ambulatory patients (≥18 years) with mild-to-moderate COVID-19 at high risk for progression to severe disease who have no other treatment options*, the IDSA guideline panel suggests molnupiravir initiated within five days of symptom onset rather than no molnupiravir. (Conditional recommendation†, Low certainty of evidence)
- Remarks:
- Patients who will most likely benefit from antivirals are those with risk factors for progression to severe disease (e.g., elderly, those with high-risk comorbidities, incomplete vaccination status, or immunocompromised).
- Those without risk factors are less likely to benefit
Patients who put a higher value on the putative mutagenesis, adverse events, or reproductive concerns and a lower value on the uncertain benefits would reasonably decline molnupiravir. - Patients with mild-to-moderate COVID-19 who are at high risk of progression to severe disease admitted to the hospital for reasons other than COVID-19 may also receive molnupiravir.
- Molnupiravir is not authorized under the FDA EUA for use in patients <18 years because it may affect bone and cartilage growth.
- Molnupiravir is not recommended under the FDA EUA for use during pregnancy.
- Molnupiravir is not authorized under the FDA EUA for pre-exposure or post-exposure prevention of COVID-19 or for initiation of treatment in patients hospitalized due to COVID-19 because benefit of treatment has not been observed in individuals when treatment is started after hospitalization due to COVID-19.
*Other options for treatment and management of ambulatory patients include nirmatrelvir/ritonavir, three-day treatment with remdesivir, Patient-specific factors (e.g., symptom duration, renal function, drug interactions) as well as product availability should drive decision-making regarding choice of agent. Data for combination treatment do not exist in this setting.
†The guideline panel concluded that the desirable effects outweigh the undesirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.

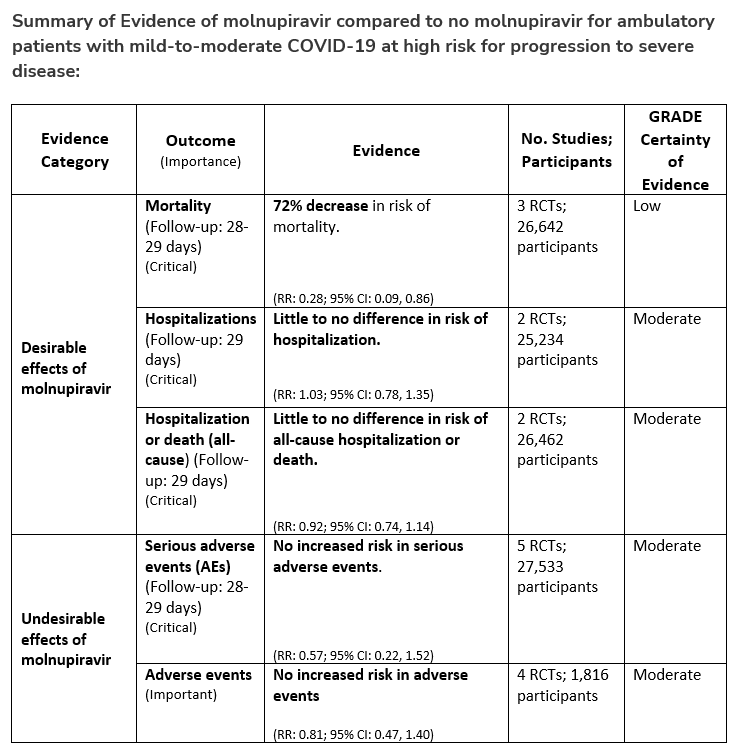
Section last reviewed and updated 6/30/2022
Last literature search conducted 5/31/2022
Recommendation 29:
In hospitalized patients with COVID-19, the IDSA panel recommends against colchicine for treatment of COVID-19. (Strong recommendation, Moderate certainty of evidence)
Recommendation 30:
In ambulatory persons with COVID-19, the IDSA panel suggests against colchicine for treatment of COVID-19. (Conditional recommendation††, Moderate certainty of evidence)
††The guideline panel concluded that the undesirable effects outweigh the desirable effects, though uncertainty still exists, and most informed people would choose the suggested course of action, while a substantial number would not.
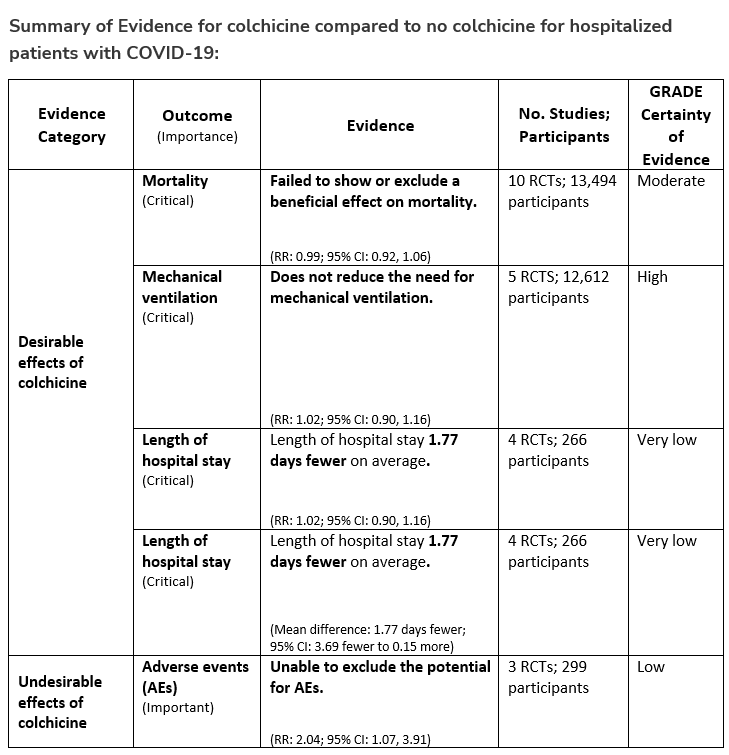
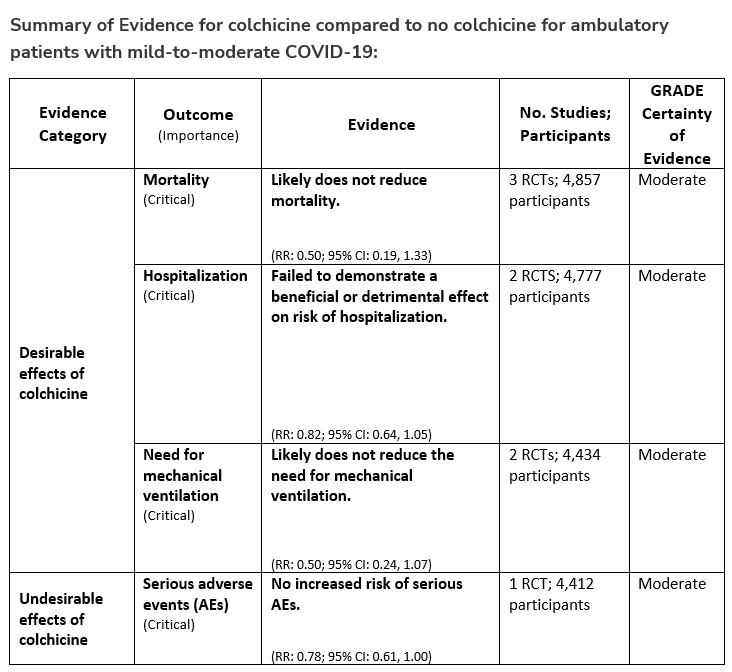
Section last reviewed and updated 5/4/2023
Last literature search conducted 3/31/2023
Recommendation 31:
In hospitalized patients with severe* COVID-19, the IDSA guideline panel suggests against the routine use of anakinra. (Conditional recommendation, Low certainty of evidence).
*Severe illness is defined as patients with SpO2 ≤94% on room air, including patients on supplemental oxygen.
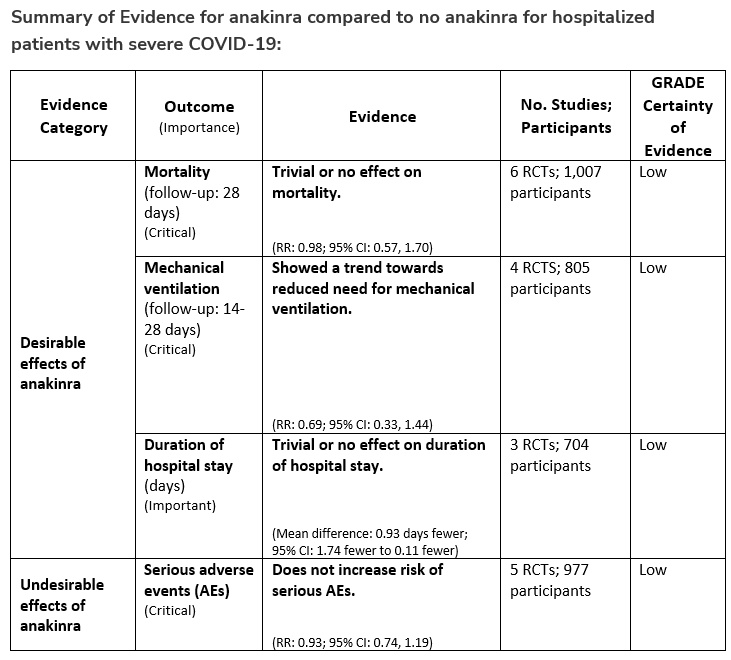
April 11, 2023
Convalescent Plasma (PDF): A new recommendation was developed against the routine use of convalescent plasma among immunocompromised patients hospitalized with COVID-19. Additionally, this section includes updated remarks for the existing recommendation on the use of convalescent plasma for ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease who have no other treatment options.
This update has been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
March 14, 2023
Version 10.2.1 has been released and includes updated evidence summaries and clarified remarks on the use of molnupiravir. No changes have been made to the current recommendation.
January 20, 2023
The following recommendation were updated based on newly available literature and approvals. They were provided here for immediate use and were later integrated into the website on February 8, 2023 as part of Version 10.2.0.
Neutralizing Antibodies for Pre-Exposure Prophylaxis: A remark was added to the recommendation regarding resistance of tixagevimab/cilgavimab (Evusheld) in the US. The agent has an Emergency Use Authorization by the US FDA and may be used in other parts of the world where the circulating COVID-19 variants may still be susceptible to it.
Neutralizing Antibodies for Post-Exposure Prophylaxis: This recommendation was retired and replaced with a statement mentioning in vitro resistance of casirivimab/imdevimab to circulating strains of COVID-19 in the US.
Neutralizing Antibodies for Treatment: This recommendation was retired and replaced with a statement mentioning that the US FDA withdrew Emergency Use Authorization for bebtelovimab, the one anti-SARS CoV-2 neutralizing antibody product that had retained in vitro activity against most previously circulating SARS-CoV-2 variants, leaving no available neutralizing antibody product in the United Sates for treatment of COVID-19.
This update has been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
November 21, 2022
Version 10.1.1 has been released and includes updates to Figure 7, Figure 8, and Figure 9.
November 15, 2022
Version 10.1.0 has been released and includes the following:
Inhaled Corticosteroids: This recommendation on the use of inhaled corticosteroids among ambulatory patients with mild-to-moderate COVID-19 has been revised.
Ivermectin: This section has been updated based on newly added literature.
This update been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
November 2, 2022
The following sections have been updated based on newly available literature and approvals. They were provided here for immediate use and were integrated into the website as part of Version 10.1.0
Ivermectin: This section has been updated based on newly added literature.
Inhaled Corticosteroids: This recommendation on the use of inhaled corticosteroids among ambulatory patients with mild-to-moderate COVID-19 has been revised.
This update been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
October 18, 2022
Version 10.0.1 has been released and includes minor corrections to Table 22 and Table 35.
August 30, 2022
The following sections were added/revised based on newly available literature and/or approvals. They were provided here for immediate use and have now been integrated into the website as part of Version 10.0.0.
Ivermectin: Revised recommendations on the use of ivermectin in hospitalized and ambulatory persons with COVID-19.
Colchicine: New recommendations on the use of colchicine in hospitalized and ambulatory persons with COVID-19.
Additionally, three new narrative sections have been developed:
How to Approach a Patient when Considering Pharmacologic Treatments for COVID-19
Bacterial Co-Infections and Antibiotic Use
This update been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
July 12, 2022
Recommendations on the use of ivermectin in hospitalized and ambulatory persons with COVID-19 were revised. These revised recommendations were provided for immediate use and were later integrated into the website on August 30, 2022, as part of Version 10.0.0. This update has been endorsed by the Pediatric Infectious Diseases Society and the Society for Healthcare Epidemiology of America.
July 7, 2022
Two new recommendations on the use of colchicine in hospitalized and ambulatory persons with COVID-19 were developed. These new recommendations were provided for immediate use and were later integrated into the website on August 30, 2022, as part of Version 10.0.0. This update has been endorsed by the Pediatric Infectious Diseases Society and the Society for Healthcare Epidemiology of America.
June 29, 2022
Version 9.0.1 has been released and includes a footnote regarding ambulatory patients receiving convalescent plasma who have no other treatment options.
June 10, 2022
Version 9.0.0 has been released and includes the following:
Famotidine: New recommendation on the use of famotidine in ambulatory patients with mild-to-moderate COVID-19; revised recommendation on the use of famotidine in hospitalized patients with severe COVID-19.
Neutralizing Antibodies for Pre- and Post-Exposure Prophylaxis: Revised recommendation on the use of tixagevimab/cilgavimab as pre-exposure prophylaxis in moderately or severely immunocompromised individuals at increased risk for inadequate immune response to COVID-19 vaccine, OR for persons for whom the COVID-19 vaccine is not recommended due to a documented serious adverse reaction to the vaccine; revised recommendation on the use of casirivimab/imdevimab as post-exposure prophylaxis for persons exposed to COVID-19 at high risk of progression to severe disease.
Neutralizing Antibodies for Treatment: Revised recommendation on the use of monoclonal antibodies in ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease.
Janus Kinase Inhibitors (Baricitinib): Revised recommendation on the use of baricitinib with corticosteroids for hospitalized adults with severe COVID-19.
This update has been endorsed by the Society for Healthcare Epidemiology of America.
May 27, 2022
The following recommendation sections were added/revised based on newly available literature and/or approvals. They were provided here for immediate use and were later integrated into the website on June 10, 2022 as part of Version 9.0.0. These updates have been endorsed by the Society for Healthcare Epidemiology of America.
Famotidine: New recommendation on the use of famotidine in ambulatory patients with mild-to-moderate COVID-19; revised recommendation on the use of famotidine in hospitalized patients with severe COVID-19.
Neutralizing Antibodies for Pre- and Post-Exposure Prophylaxis: Revised recommendation on the use of tixagevimab/cilgavimab as pre-exposure prophylaxis in moderately or severely immunocompromised individuals at increased risk for inadequate immune response to COVID-19 vaccine, OR for persons for whom the COVID-19 vaccine is not recommended due to a documented serious adverse reaction to the vaccine; revised recommendation on the use of casirivimab/imdevimab as post-exposure prophylaxis for persons exposed to COVID-19 at high risk of progression to severe disease.
Neutralizing Antibodies for Treatment: Revised recommendation on the use of monoclonal antibodies in ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease.
May 10, 2022
A recommendation on the use of baricitinib with corticosteroids for hospitalized adults with severe COVID-19 was revised. This revised recommendation was provided for immediate use and was later integrated into the website on June 10, 2022 as part of Version 9.0.0. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
March 23, 2022
Version 8.0.0 has been released and includes new recommendations on the use of inhaled glucocorticoids in ambulatory patients with mild-to-moderate COVID-19 and bebtelovimab in ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
March 18, 2022
A new recommendation was developed on the use of inhaled corticosteroids in ambulatory patients with mild-to-moderate COVID-19. This new recommendation was originally provided for immediate use and was later integrated into the website on March 23, 2022 as part of Version 8.0.0.
March 14, 2022
Version 7.0.1 has been released and includes an update to the dosing for tixagevimab/cilgavimab as pre-exposure prophylaxis for moderately or severely immunocompromised individuals at increased risk for inadequate immune response to COVID-19 vaccine OR for whom COVID-19 vaccine is not recommended due to a documented serious adverse reaction to the vaccine.
March 11, 2022
A new recommendation was developed on the use of bebtelovimab in ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease. This new recommendation was originally provided for immediate use and was later integrated into the website on March 23, 2022 as part of Version 8.0.0.
March 9, 2022
Version 7.0.0 has been released and includes new recommendations on the use of lopinavir/ritonavir for individuals exposed to or with COVID-19, a revised recommendation on the use of convalescent plasma in ambulatory patients with mild-to-moderate COVID-19, and a revised recommendation for the use of remdesivir in patients (ambulatory or hospitalized) with mild-to-moderate COVID-19 at high risk of progression to severe disease. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
February 22, 2022
Two new recommendations were developed on the use of lopinavir/ritonavir (prophylaxis for persons exposed to SARS-CoV-2; treatment for ambulatory patients with mild-to-moderate COVID-19). This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society. These new recommendations were originally provided for immediate use and were later integrated into the website on March 9, 2022 as part of Version 7.0.0.
February 16, 2022
A revised recommendation was released on the use of remdesivir in patients (ambulatory or hospitalized) with mild-to-moderate COVID at high risk for progression to severe disease. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society. This revised recommendation was originally provided for immediate use and was later integrated into the website on March 9, 2022 as part of Version 7.0.0.
February 8, 2022
A new recommendation was released on the use of convalescent plasma in ambulatory patients with mild-to-moderate COVID-19 at high risk for progression to severe disease with no other treatment options. This recommendation was originally provided for immediate use and was later integrated into the website on March 9, 2022 as part of Version 7.0.0.
February 3, 2022
Version 6.0.2 has been released and includes an update to the evidence profile for nirmatrelvir/ritonavir in ambulatory patients (outcome of COVID-19 related hospitalizations).
January 18, 2022
Version 6.0.1 as been released and includes endorsement from the Society for Infectious Diseases Pharmacists.
January 12, 2022
Version 6.0.0 as been released and includes new recommendations on the use of remdesivir for ambulatory patients, tixagevimab/cilgavimab for pre-exposure prophylaxis, nirmatrelvir/ritonavir in ambulatory patients, and molnupiravir for ambulatory patients. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
December 30, 2021
A new recommendation was released on the use of molnupiravir for ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease who have no other treatment options. This recommendation was originally provided for immediate use and was later integrated into the website on January 12, 2022 as part of Version 6.0.0.
December 24, 2021
The following recommendation sections were added based on newly available literature and/or approvals. They were provided here for immediate use and were later integrated into the website on January 12, 2022 as part of Version 6.0.0.
Remdesivir: New recommendation on the use of remdesivir for ambulatory patients
Neutralizing Antibodies for Prophylaxis: New recommendation on the use of tixagevimab/cilgavimab for pre-exposure prophylaxis in adults at increased risk for inadequate immune response to COVID-19 vaccine or for whom COVID-19 vaccine is not recommended
Oral Antivirals: New recommendation on the use of nirmatrelvir/ritonavir in ambulatory patients with mild to moderate COVID-19 at high risk for progression to severe disease
The language in the above section has been updated, with "nirmatrelvir/ritonavir" replacing "oral antivirals". Additionally, information on the use of brand name as well as use in patients hospitalized for reasons other than COVID-19 has been added.
November 18, 2021
Version 5.6.0 as been released and includes revised recommendations on the use of convalescent plasma in hospitalized and ambulatory patients with COVID-19; this update has been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
November 9, 2021
Version 5.5.3 has been released and contains a correction to the analysis for the outcome of mortality in ambulatory patients treated with fluvoxamine.
November 5, 2021
Version 5.5.2 has been released and includes updated literature for the use of fluvoxamine in ambulatory patients.
November 1, 2021
Version 5.5.1 has been released and includes endorsement from the Society of Infectious Diseases Pharmacists.
October 27, 2021
Version 5.5.0 has been released and contains a new recommendation on the use of fluvoxamine in ambulatory patients. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Pediatric Infectious Diseases Society.
October 18, 2021
Version 5.4.1 has been released and contains a minor correction to the neutralizing antibodies section. Subcutaneous has been removed to the dosing for bamlanivimab/etesevimab.
October 15, 2021
Version 5.4.0 has been released and includes revised remarks and a new evidence profile for the use of baricitinib for critically ill patients requiring invasive mechanical ventilation. This update has been endorsed by the Society of Infectious Diseases Pharmacists.
October 1, 2021
Version 5.3.1 has been released and contains a correction to the certainty of evidence for the new recommendation on the use of hydroxychloroquine as post-exposure prophylaxis. This recommendation has a moderate--not low--certainty of evidence.
September 30, 2021
Version 5.3.0 has been released and includes a new recommendation on the use of hydroxychloroquine as post-exposure prophylaxis. This update has been endorsed by the Society for Healthcare Epidemiology of America and the Society of Infectious Diseases Pharmacists.
September 24, 2021
Version 5.2.1 has been released and features existing recommendations on the use of neutralizing SARS-CoV-2 antibodies separated by prophylaxis vs. treatment.
September 21, 2021
Version 5.2.0 has been released and includes a new recommendation on the use of sarilumab. This update has been endorsed by the Society for Healthcare Epidemiology of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists.
September 14, 2021
Version 5.1.2 has been released and includes endorsement from the Society of Infectious Diseases Pharmacists.
September 3, 2021
Version 5.1.1 has been released and includes endorsement from the Pediatric Infectious Diseases Society.
August 27, 2021
Version 5.1.0 has been released and includes revised remarks for the use of baricitinib and new recommendations on the use of tofacitinib.
August 25, 2021
Version 5.0.0 has been released and includes new and updated recommendations for neutralizing antibodies and ivermectin. This update has been endorsed by the Society for Healthcare Epidemiology of America.
June 25, 2021
Version 4.4.1 has been released and includes endorsement from the Pediatric Infectious Diseases Society.
June 23, 2021
Version 4.4.0 has been released and includes updated recommendations and literature summary on neutralizing antibodies.
June 3, 2021
Version 4.3.0 has been released and contains a new recommendation on the use of remdesivir.
May 3, 2021
Version 4.2.1 has been released and contains endorsement from the Pediatric Infectious Diseases Society.
April 14, 2021
April 5, 2021
Version 4.1.2 has been released and contains a revision to the number of studies included in Table 9 in the section on remdesivir.
March 18, 2021
Version 4.1.1 has been released and contains a revision to the number of studies found for ivermectin.
March 5, 2021
Version 4.1.0 has been released and contains a new recommendation on the use of bamlanivimab with etesevimab among ambulatory patients.
February 22, 2021
Version 4.0.0 has been released and contains a revised recommendation on the use of tocilizumab.
February 18, 2021
Version 3.10.0 has been released and includes additional information on study eligibility for ivermectin.
February 10, 2021
Version 3.9.0 has been released and contains an updated literature review for tocilizumab.
February 5, 2021
Version 3.8.0 has been released and includes two new recommendations on the use of ivermectin.
February 3, 2021
Version 3.7.0 has been released and includes two new recommendations on the use of neutralizing monoclonal antibodies.
January 8, 2021
Version 3.6.0 has been realeased and includes new recommendations on the use of baricitinib and an updated literature review on hydroxychloroquine.
December 2, 2020
Version 3.5.1 has been released and includes endorsement from the Pediatric Infectious Diseases Society.
November 22, 2020
Version 3.5.0 has been released and includes revisions to the sections on lopinavir/ritonavir, tocilizumab, and remdesivir.
November 18, 2020
Version 3.4.0 has been released and contains a new recommendation on the use of bamlanivimab.
September 25, 2020
Version 3.3.0 has been released and contains revised and new recommendations for the use of dexamethasone and a revised recommendation against the routine use of tocilizumab.
September 21, 2020
Version 3.2.1 has been released and includes endorsement from the Pediatric Infectious Diseases Society.
September 15, 2020
Version 3.2.0 has been released and contains a new recommendation on the use of remdesivir in patients with more moderate disease.
September 4, 2020
Version 3.1.0 has been released and contains additional information on convalescent plasma as well new and updated narrative summaries of treatments undergoing evaluation.
August 28, 2020
Version 3.0.1 has been released and includes endorsement from the Pediatric Infectious Diseases Society.
August 20, 2020
Version 3.0.0 of the guideline has been released and contains revised recommendations on hydroxychloroquine and hydroxychloroquine plus azithromycin.
June 25, 2020
Version 2.1.0 of the guideline has been released and includes revised recommendations on corticosteroids.
June 22, 2020
Version 2.0.0 of the guideline has been released and contains:
Revised recommendations on hydroxychloroquine and hydroxychloroquine plus azithromycin
Revised recommendations for convalescent plasma for treatment of COVID-19
New recommendations on the use of remdesivir
New recommendations for famotidine (not addressed in versions 1.0.0-1.0.4)
April 21, 2020
Version 1.0.4 of the guideline has been released.